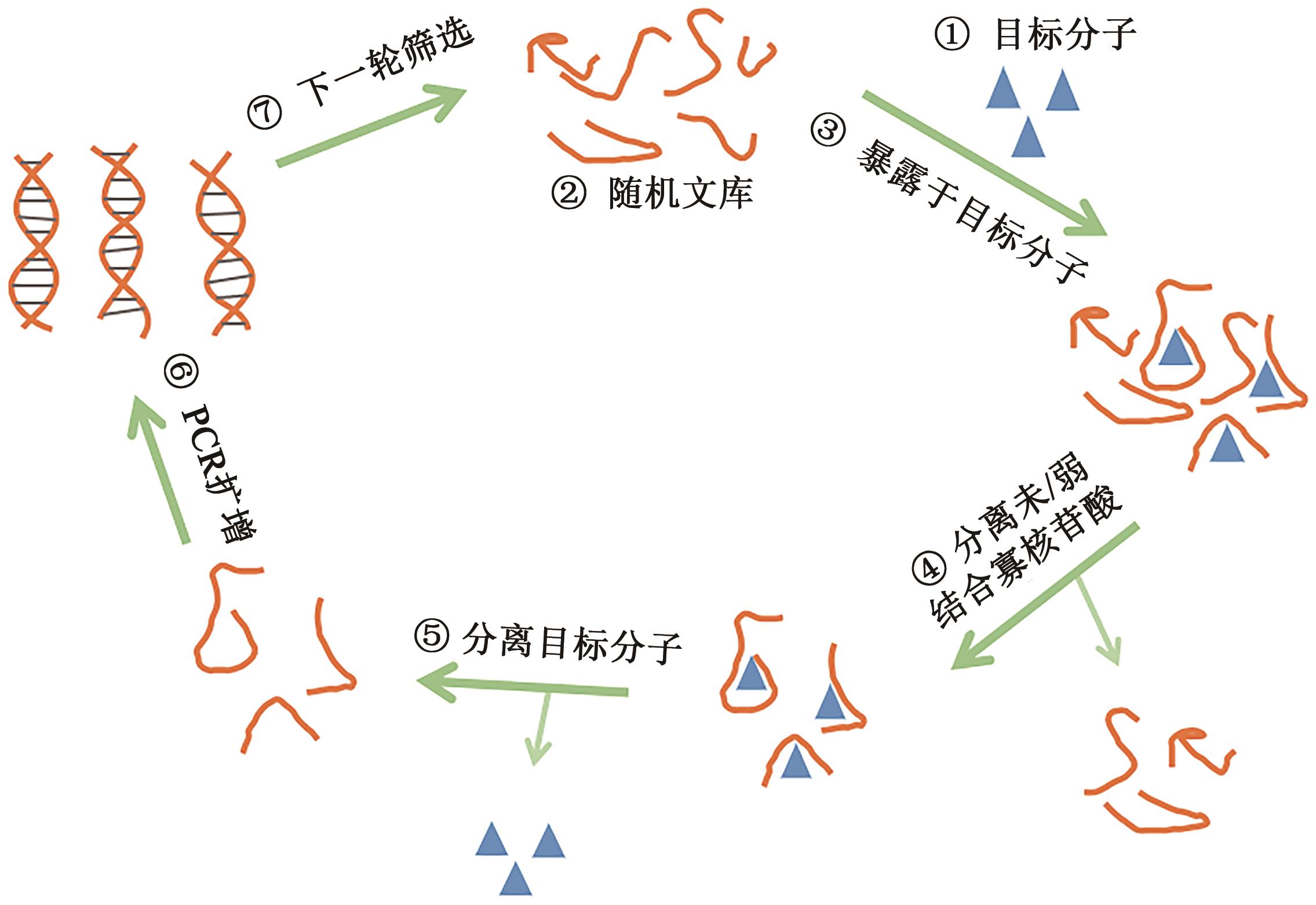
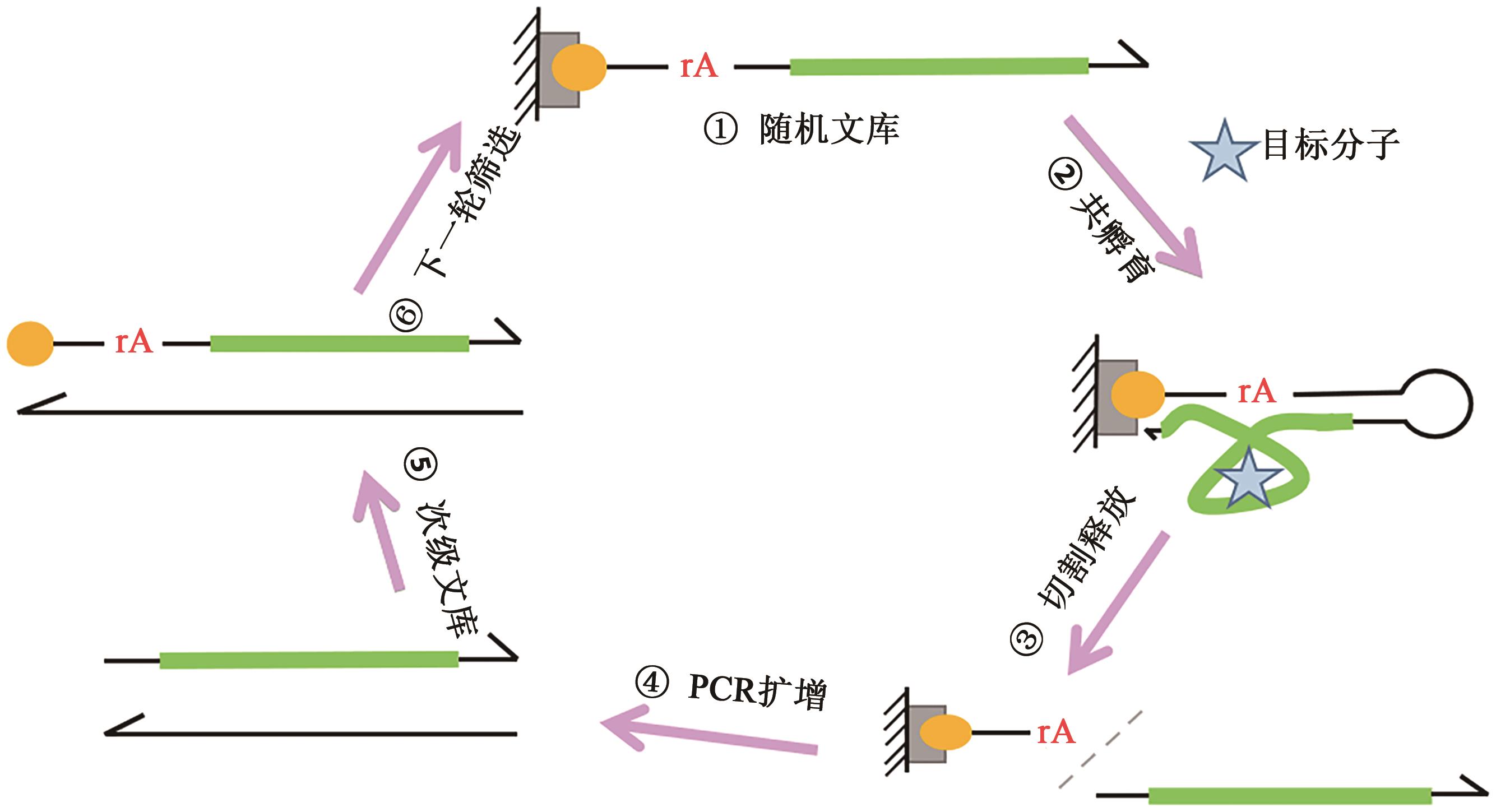
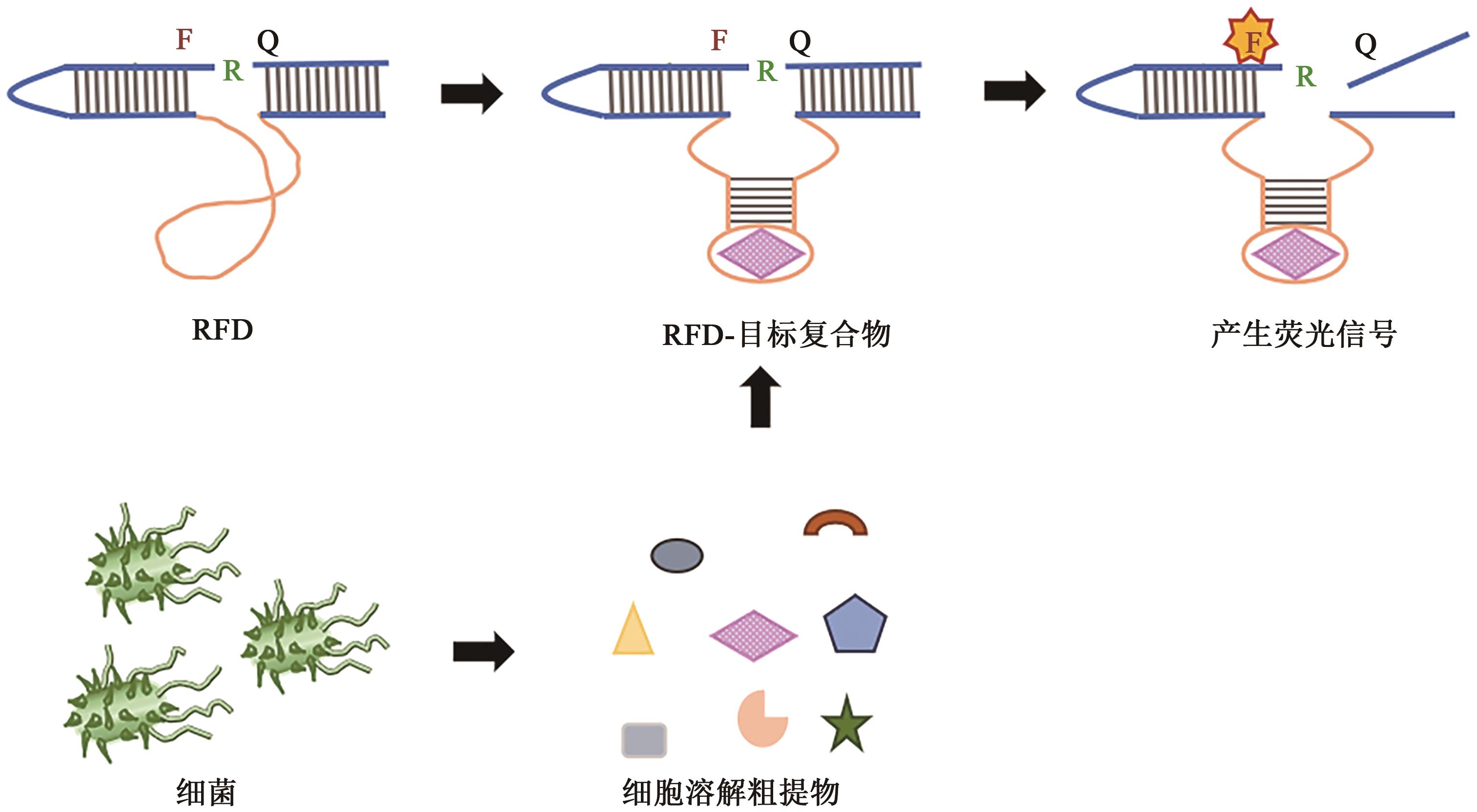
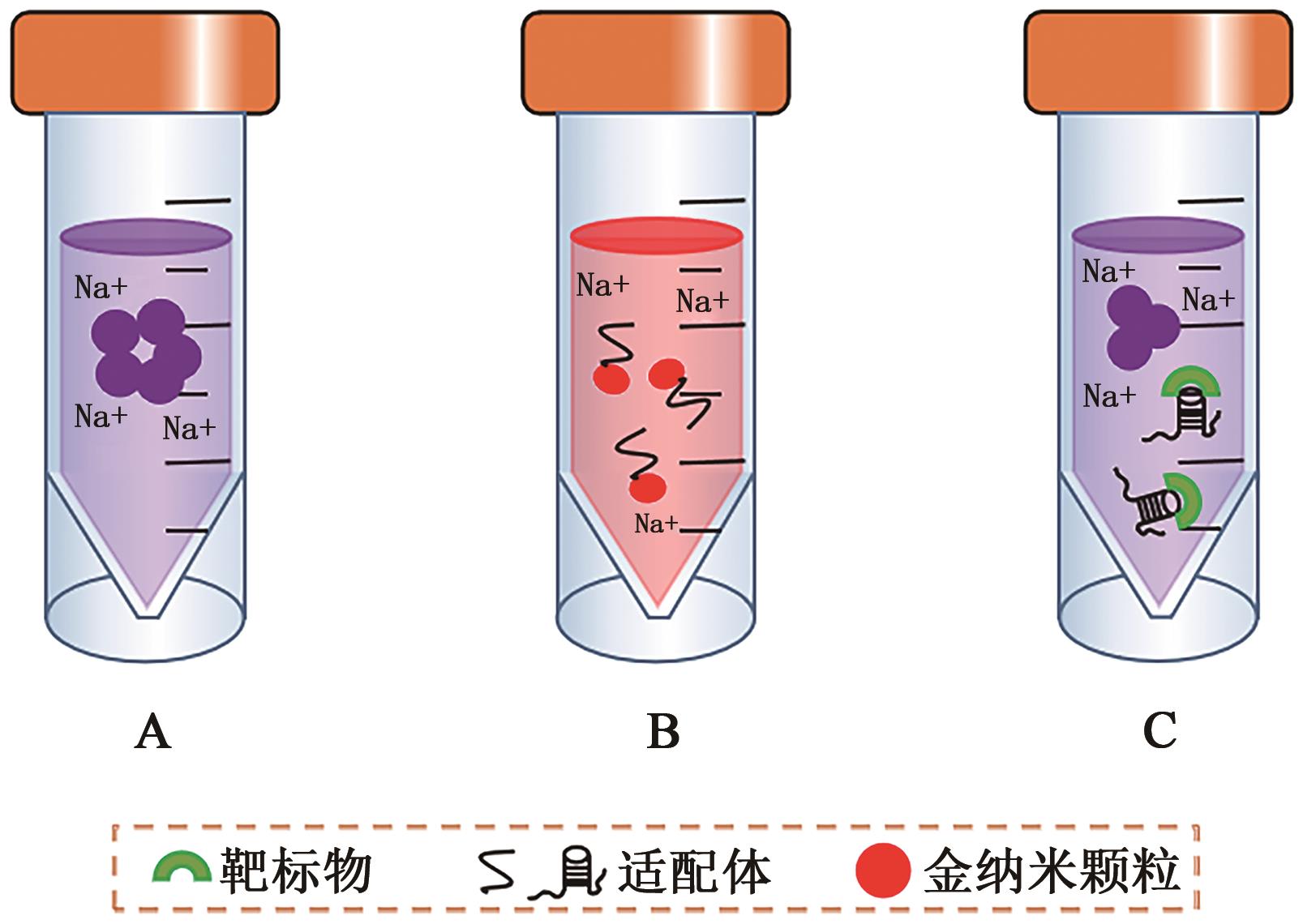
Current Biotechnology ›› 2023, Vol. 13 ›› Issue (1): 30-38.DOI: 10.19586/j.2095-2341.2022.0089
• Special Forum on Food Safety Detection • Previous Articles Next Articles
Research Progress of Functional Nucleic Acid Used in Pathogenic Bacteria Detection
Wenzhuo ZHAO( ), Chengxun LI, Zuojian HU, Hongxiu YU(
), Chengxun LI, Zuojian HU, Hongxiu YU( )
)
- Institutes of Biomedical Sciences & Shanghai Stomatological Hospital,Fudan University,Shanghai 200032,China
-
Received:2022-05-26Accepted:2022-08-18Online:2023-01-25Published:2023-02-07 -
Contact:Hongxiu YU
功能核酸用于致病菌检测的研究进展
- 复旦大学生物医学研究院&附属口腔医院,上海 200032
-
通讯作者:余红秀 -
作者简介:赵文卓 E-mail: 21211510023@m.fudan.edu.cn; -
基金资助:国家重点研发计划项目(2021YFC2701802);上海市口腔医院科技创新人才培养计划(SSH-2022-KJCX-A01)
CLC Number:
Cite this article
Wenzhuo ZHAO, Chengxun LI, Zuojian HU, Hongxiu YU. Research Progress of Functional Nucleic Acid Used in Pathogenic Bacteria Detection[J]. Current Biotechnology, 2023, 13(1): 30-38.
赵文卓, 李成勋, 胡作建, 余红秀. 功能核酸用于致病菌检测的研究进展[J]. 生物技术进展, 2023, 13(1): 30-38.
share this article
| 检测方法 | 适用场所 | 优缺点 | 检测对象 | 检测元件 | 检测限/(CFU·mL-1) | 参考文献 |
|---|---|---|---|---|---|---|
| 荧光法 | 实验室、机构检测 | 检出限较低,但需要相关仪器设备,成本高 | 苏云金芽孢杆菌(Bacillus thuringiensis) | 适配体 | 103 | [ |
| 副溶血弧菌和鼠伤寒沙门氏菌(Vibrio parahaemolyticus and Salmonella typhimurium) | 适配体 | 5×103 | [ | |||
| 大肠杆菌ATCC 8739(Escherichia coli) | 适配体 | 3 | [ | |||
| 鼠伤寒沙门氏菌(Salmonella typhimurium) | 适配体 | 50 | [ | |||
| 大肠杆菌(Escherichia coli) | RCDs | 103 | [ | |||
| 肺炎克雷伯菌(Klebsiella Pneumoniae) | RCDs | 105 | [ | |||
| 鳗弧菌(Vibrio anguillarum) | RCDs | 4×103 | [ | |||
| 比色法 | 现场检测、实验室、机构检测 | 直观快速,不需要仪器支撑,成本低,但结果不易量化 | 金黄色葡萄球菌(Staphylococcus aureus) | 适配体 | 108 | [ |
| 阪崎肠杆菌(Cronobacter sakazakii) | 适配体 | 7.1×103 | [ | |||
| 幽门螺旋杆菌(Helicobacter pylori) | RCDs | 104 | [ | |||
| 金黄色葡萄球菌(Staphylococcus aureus) | RCDs | 105 | [ | |||
| 电化学法 | 现场检测、实验室、机构检测 | 灵敏度高,结果直观,但需要相关电子设备,成本较高 | 金黄色葡萄球菌(Staphylococcus aureus) | 适配体 | 41 | [ |
| 金黄色葡萄球菌(Staphylococcus aureus) | 适配体 | 39(缓冲液) 414(自来水) | [ | |||
| 大肠杆菌(Escherichia coli) | RCDs | 103 | [ |
Table 1 Performance comparison of biosensors for detecting pathogenic bacteria based on functional nucleic acids
| 检测方法 | 适用场所 | 优缺点 | 检测对象 | 检测元件 | 检测限/(CFU·mL-1) | 参考文献 |
|---|---|---|---|---|---|---|
| 荧光法 | 实验室、机构检测 | 检出限较低,但需要相关仪器设备,成本高 | 苏云金芽孢杆菌(Bacillus thuringiensis) | 适配体 | 103 | [ |
| 副溶血弧菌和鼠伤寒沙门氏菌(Vibrio parahaemolyticus and Salmonella typhimurium) | 适配体 | 5×103 | [ | |||
| 大肠杆菌ATCC 8739(Escherichia coli) | 适配体 | 3 | [ | |||
| 鼠伤寒沙门氏菌(Salmonella typhimurium) | 适配体 | 50 | [ | |||
| 大肠杆菌(Escherichia coli) | RCDs | 103 | [ | |||
| 肺炎克雷伯菌(Klebsiella Pneumoniae) | RCDs | 105 | [ | |||
| 鳗弧菌(Vibrio anguillarum) | RCDs | 4×103 | [ | |||
| 比色法 | 现场检测、实验室、机构检测 | 直观快速,不需要仪器支撑,成本低,但结果不易量化 | 金黄色葡萄球菌(Staphylococcus aureus) | 适配体 | 108 | [ |
| 阪崎肠杆菌(Cronobacter sakazakii) | 适配体 | 7.1×103 | [ | |||
| 幽门螺旋杆菌(Helicobacter pylori) | RCDs | 104 | [ | |||
| 金黄色葡萄球菌(Staphylococcus aureus) | RCDs | 105 | [ | |||
| 电化学法 | 现场检测、实验室、机构检测 | 灵敏度高,结果直观,但需要相关电子设备,成本较高 | 金黄色葡萄球菌(Staphylococcus aureus) | 适配体 | 41 | [ |
| 金黄色葡萄球菌(Staphylococcus aureus) | 适配体 | 39(缓冲液) 414(自来水) | [ | |||
| 大肠杆菌(Escherichia coli) | RCDs | 103 | [ |
| 1 | 白小莲,爱军.生物传感器在食源性致病菌大肠杆菌O157:H7检测中的应用进展[J].生物技术进展,2021,11(3):269-278. |
| 2 | WILCOCK A, PUN M, KHANONA J, et al.. Consumer attitudes,knowledge and behaviour: a review of food safety issues[J].Trends Food Sci. Tech., 2004, 15(2): 56-66. |
| 3 | 常仁亮.制定及应用食品微生物标准的原则CAC/GL21-1997[J]. 现代渔业信息,1999(7):17-19. |
| 4 | 中华人民共和国食品安全法(节选)[J]. 粮食科技与经济, 2022, 47(S1): 10-15. |
| 5 | TAIT E, PERRY J D, STANFORTH S P, et al.. Use of volatile compounds as a diagnostic tool for the detection of pathogenic bacteria[J]. Trac-Trend Anal. Chem., 2014, 53: 117-125. |
| 6 | BHARDWAJ N, BHARDWAJ S K, NAYAK M K, et al.. Fluorescent nanobiosensors for the targeted detection of foodborne bacteria[J]. Trac-Trend Anal. Chem., 2017, 97: 120-135. |
| 7 | 吴一凡,林晟豪,许文涛.小分子靶标的核糖开关生物传感器研究进展[J]. 生物技术进展, 2022, 12(2): 168-175. |
| 8 | LI Y, LU Y. Functional nucleic acids for analytical applications[M]. New York: Springer, 2009. |
| 9 | 许文涛,杨敏,朱龙佼,等.功能核酸概念的内涵与外延[J]. 生物技术进展, 2021, 11(4): 446-454. |
| 10 | 王琦,颜春蕾,高洪伟,等.基于核酸适配体传感器检测食品致病菌的研究进展[J]. 生物技术通报, 2020, 36(11): 245-258. |
| 11 | YOU K M, LEE S H, IM A, et al.. Aptamers as functional nucleic acids: in vitro selection and biotechnological applications[J]. Biotechnol. Bioproc. E., 2003, 8(2): 64-75. |
| 12 | MIYAKE Y, TOGASHI H, TASHIRO M, et al.. Mercury(II)-mediated formation of thymine-Hg-II-thymine base pairs in DNA duplexes[J]. J. Am. Chem. Soc., 2006, 128(7): 2172-2173. |
| 13 | ELLINGTON A D, SZOSTAK J W. Invitro selection of RNA molecules that bind specific ligands[J]. Nature, 1990, 346(6287): 818-822. |
| 14 | TUERK C, GOLD L. Systematic evolution of ligands by exponential enrichment-RNA ligands to bacteriophage-T4 DNA-polymerase[J]. Science, 1990, 249(4968): 505-510. |
| 15 | BRUNO J G. In vitro selection of DNA to chloroaromatics using magnetic microbead-based affinity separation and fluorescence detection[J]. Biochem. Bioph. Res. Co., 1997, 234(1): 117-120. |
| 16 | MENDONSA S D, BOWSER M T. In vitro evolution of functional DNA using capillary electrophoresis[J]. J. Am. Chem. Soc., 2004, 126(1): 20-21. |
| 17 | MENDONSA S D, BOWSER M T. In vitro selection of high-affinity DNA ligands for human IgE using capillary electrophoresis[J]. Anal. Chem., 2004, 76(18): 5387-5392. |
| 18 | DANIELS D A, CHEN H, HICKE B J, et al.. A tenascin-C aptamer identified by tumor cell SELEX: systematic evolution of ligands by exponential enrichment[J]. Proc. Natl. Acad. Sci. USA, 2003, 100(26): 15416-15421. |
| 19 | BEREZOVSKI M, MUSHEEV M, DRABOVICH A, et al.. Non-SELEX selection of aptamers[J]. J. Am. Chem. Soc., 2006, 128(5): 1410-1411. |
| 20 | BEREZOVSKI M V, MUSHEEV M U, DRABOVICH A P, et al.. Non-SELEX: selection of aptamers without intermediate amplification of candidate oligonucleotides[J]. Nat. Protoc., 2006, 1(3): 1359-1369. |
| 21 | CHEN Z H, ZENG Z H, WAN Q Y, et al.. Targeted immunotherapy of triple-negative breast cancer by aptamer-engineered NK cells[J/OL]. Biomaterials, 2022, 280:121259[2021-11-15]. |
| 22 | KEIJZER J F, FIRET J, ALBADA B. Site-selective and inducible acylation of thrombin using aptamer-catalyst conjugates[J]. Chem. Commun., 2021, 57(96): 12960-12963. |
| 23 | IKANOVIC M, RUDZINSKI W E, BRUNO J G, et al.. Fluorescence assay based on aptamer-quantum dot binding to Bacillus thuringiensis spores[J]. J. Fluoresc., 2007, 17(2): 193-199. |
| 24 | DUAN N, WU S, YU Y, et al.. A dual-color flow cytometry protocol for the simultaneous detection of Vibrio parahaemolyticus and Salmonella typhimurium using aptamer conjugated quantum dots as labels[J]. Anal. Chim. Acta, 2013, 804: 151-158. |
| 25 | JIN B, WANG S, LIN M, et al.. Upconversion nanoparticles based FRET aptasensor for rapid and ultrasenstive bacteria detection[J]. Biosens. Bioelectron., 2017, 90: 525-533. |
| 26 | WANG R J, XU Y, ZHANG T, et al.. Rapid and sensitive detection of Salmonella typhimurium using aptamer-conjugated carbon dots as fluorescence probe[J]. Anal. Methods, 2015, 7(5): 1701-1706. |
| 27 | YOUSEFI H, ALI M M, SU H M, et al.. Sentinel wraps: real-time monitoring of food contamination by printing DNAzyme probes on food packaging[J]. ACS Nano, 2018, 12(4): 3287-3294. |
| 28 | ALI M M, SLEPENKIN A, PETERSON E, et al.. A simple DNAzyme-based fluorescent assay for Klebsiella pneumoniae [J]. Chembiochem, 2019, 20(7): 906-910. |
| 29 | GU L, YAN W, WU H, et al.. Selection of DNAzymes for sensing aquatic bacteria: Vibrio anguillarum [J]. Anal. Chem., 2019, 91(12): 7887-7893. |
| 30 | CHANG T, WANG L, ZHAO K, et al.. Duplex identification of Staphylococcus aureus by aptamer and gold nanoparticles[J]. J. Nanosci. Nanotechnol., 2016, 16(6): 5513-5519. |
| 31 | KIM H S, KIM Y J, CHON J W, et al.. Two-stage label-free aptasensing platform for rapid detection of Cronobacter sakazakii in powdered infant formula[J]. Sensor. Actuat. B-Chem., 2017, 239: 94-99. |
| 32 | ALI M M, WOLFE M, TRAM K, et al.. ADNAzyme-based colorimetric paper sensor for Helicobacter pylori [J]. Angew. Chem. Int. Edit., 2019, 58(29): 9907-9911. |
| 33 | ALI M M, SILVA R, WHITE D, et al.. A lateral flow test for Staphylococcus aureus in nasal mucus using a new DNAzyme as the recognition element[J/OL]. Angew. Chem. Int. Ed. Engl., 2022, 61(3): e202112346[2021-11-23]. . |
| 34 | LIAN Y, HE F, WANG H, et al.. A new aptamer/graphene interdigitated gold electrode piezoelectric sensor for rapid and specific detection of Staphylococcus aureus [J]. Biosens. Bioelectron., 2015, 65: 314-319. |
| 35 | NGUYEN T T, KIM E R, GU M B. A new cognate aptamer pair-based sandwich-type electrochemical biosensor for sensitive detection of Staphylococcus aureus [J/OL]. Biosens. Bioelectron., 2022, 198: 113835[2021-11-24]. . |
| 36 | PANDEY R, CHANG D R, SMIEJA M, et al.. Integrating programmable DNAzymes with electrical readout for rapid and culture-free bacterial detection using a handheld platform[J]. Nat. Chem., 2021, 13(9): 895-901. |
| 37 | LIU J, CAO Z, LU Y. Functional nucleic acid sensors[J]. Chem.Rev., 2009, 109(5): 1948-1998. |
| 38 | BREAKER R R, JOYCE G F. A DNA enzyme that cleaves RNA[J]. Chemi. Biol., 1994, 1(4): 223-229. |
| 39 | KIM K S, HCHOI W, GONG S J, et al. Efficient target site selection for an RNA-cleaving DNAzyme through combinatorial library screening[J]. B. Korean Chem. Soc., 2006, 27(5): 657-662. |
| 40 | SHEN Z, WU Z, CHANG D, et al.. A catalytic DNA activated by a specific strain of bacterial pathogen[J]. Angew. Chem. Int. Edit., 2016, 55(7): 2431-2434. |
| 41 | ZHANG W, FENG Q, CHANG D, et al.. In vitro selection of RNA-cleaving DNAzymes for bacterial detection[J]. Methods, 2016, 106: 66-75. |
| 42 | GU L, YAN W, WU H, et al.. Selection of DNAzymes for sensing aquatic bacteria: vibrio anguillarum[J]. Anal. Chem., 2019, 91(12): 7887-7893. |
| 43 | SHANGGUAN J F, LI Y H, HE D G, et al.. A combination of positive dielectrophoresis driven on-line enrichment and aptamer-fluorescent silica nanoparticle label for rapid and sensitive detection of Staphylococcus aureus [J]. Analyst, 2015, 140(13): 4489-4497. |
| 44 | WANG Q Y, KANG Y J. Bioprobes based on aptamer and silica fluorescent nanoparticles for bacteria Salmonella typhimurium detection[J]. Nanoscale Res. Lett., 2016, 11(1): 1-9. |
| 45 | DUAN N, WU S J, ZHU C Q, et al.. Dual-color upconversion fluorescence and aptamer-functionalized magnetic nanoparticles-based bioassay for the simultaneous detection of Salmonella typhimurium and Staphylococcus aureus [J]. Anal. Chim. Acta, 2012, 723: 1-6. |
| 46 | WU S J, DUAN N, SHI Z, et al.. Simultaneous aptasensor for multiplex pathogenic bacteria detection based on multicolor upconversion nanoparticles labels[J]. Anal. Chem., 2014, 86(6): 3100-3107. |
| 47 | KURT H, YUCE M, HUSSAIN B, et al.. Dual-excitation upconverting nanoparticle and quantum dot aptasensor for multiplexed food pathogen detection[J]. Biosens. Bioelectron., 2016, 81: 280-286. |
| 48 | QIAN Z S, SHAN X Y, CHAI L J, et al.. A universal fluorescence sensing strategy based on biocompatible graphene quantum dots and graphene oxide for the detection of DNA[J]. Nanoscale, 2014, 6(11): 5671-5674. |
| 49 | ZHANG C, LAI C, ZENG G M, et al.. Nanoporous Au-based chronocoulometric aptasensor for amplified detection of Pb2+ using DNAzyme modified with Au nanoparticles[J]. Biosens. Bioelectron., 2016, 81: 61-67. |
| 50 | LIU D B, WANG Z, JIANG X Y. Gold nanoparticles for the colorimetric and fluorescent detection of ions and small organic molecules[J]. Nanoscale, 2011, 3(4): 1421-1433. |
| 51 | CHAN W C W, NIE S M. Quantum dot bioconjugates for ultrasensitive nonisotopic detection[J]. Science, 1998, 281(5385): 2016-2018. |
| 52 | BILAN R, FLEURY F, NABIEY I, et al.. Quantum dot surface chemistry and functionalization for cell targeting and imaging[J]. Bioconjugate Chem., 2015, 26(4): 609-624. |
| 53 | NANDI S, RITENBERG M, JELINEK R. Bacterial detection with amphiphilic carbon dots[J]. Analyst, 2015, 140(12): 4232-4237. |
| 54 | ALI M M, AGUIRRE S D, LAZIM H, et al.. Fluorogenic DNAzyme probes as bacterial indicators[J]. Angew. Chem. Int. Edit., 2011, 50(16): 3751-3754. |
| 55 | MEI S H J, LIU Z J, BRENNAN J D, et al.. An efficient RNA-cleaving DNA enzyme that synchronizes catalysis with fluorescence signaling[J]. J. Am. Chem. Soc., 2003, 125(2): 412-420. |
| 56 | LIU Z J, MEI S H J, BRENNAN J D, et al.. Assemblage of signaling DNA enzymes with intriguing metal-ion specificities and pH dependences[J]. J. Am. Chem. Soc., 2003, 125(25): 7539-7545. |
| 57 | ALI M M, AGUIRRE S D, LAZIM H, et al.. Fluorogenic DNAzyme probes as bacterial indicators[J]. Angew. Chem. Int. Ed. Engl., 2011, 50(16): 3751-3754. |
| 58 | ZHU D, LIU B, WEI G. Two-dimensional material-based colorimetric biosensors: a review[J]. Biosensors-Basel, 2021, 11(8): 259. |
| 59 | VERMA M S, ROGOWSKI J L, JONES L, et al.. Colorimetric biosensing of pathogens using gold nanoparticles[J]. Biotechnol. Adv., 2015, 33(6): 666-680. |
| 60 | LI X M, JU H Q, DING C F, et al.. Nucleic acid biosensor for detection of hepatitis B virus using 2,9-dimethyl-1,10-phenanthroline copper complex as electrochemical indicator[J]. Anal. Chim. Acta, 2007, 582(1): 158-163. |
| 61 | WANG C F, SUN X Y, SU M, et al.. Electrochemical biosensors based on antibody, nucleic acid and enzyme functionalized graphene for the detection of disease-related biomolecules[J]. Analyst, 2020, 145(5): 1550-1562. |
| 62 | 张爱萍,唐佳妮,孟瑞锋, 等.电化学法快速检测微生物的发展现状及趋势[J]. 生物技术进展, 2011, 1(5): 342-346. |
| [1] | Yue SHI, Yao HAN, Hao LI, Yansong SUN. Application Progress of Biosensors Based on Field-effect Transistors in Nucleic Acid Detection [J]. Current Biotechnology, 2025, 15(4): 597-605. |
| [2] | Yuqi YANG, Xiuxia HE. Application of Rolling Circle Amplification Technique in Electrochemical Biosensors [J]. Current Biotechnology, 2023, 13(6): 863-867. |
| [3] | Enze CHENG-CHEN, Minghong JIA, Yueying LI. Research Progress of Nucleic Acid Reference Materials of Food-borne Pathogenic Bacteria [J]. Current Biotechnology, 2023, 13(2): 195-200. |
| [4] | Doudou LEI, Runran MA, Jiabo WANG, Weijun KONG. Research Progress on New Biological Detection Technology for Pesticide Residues in Foods [J]. Current Biotechnology, 2023, 13(1): 1-10. |
| [5] | Yifan WU, Shenghao LIN, Wentao XU. Research Progress of Riboswitch Biosensors for Small Molecule Target [J]. Current Biotechnology, 2022, 12(2): 168-175. |
| [6] | Yan HU, Ling CHEN. Detection the Affinity Between PDL1 Antibodies and PDL1 Antigen Based on Biolayer Interferometry [J]. Current Biotechnology, 2021, 11(6): 795-801. |
| [7] | LU Haiqiang1, JIAO Xinya1,2, WU Siyuan2, WANG Yali2, LIU Lu2, CHENG Shumei1, ZHANG Xiao3*, SU Xiaofeng2*. Application Progress on the Digital PCR in Detection of Foodborne Pathogenic Bacteria [J]. Curr. Biotech., 2021, 11(3): 260-268. |
| [8] | BAI Xiaolian, AI Jun*. Application Progress of Biosensor in Detection of Food-borne Pathogen Escherichia coli O157∶H7 [J]. Curr. Biotech., 2021, 11(3): 269-278. |
| [9] | CHEN Shuo1, GAO Jiaqi1, WANG Di2, LONG Yan2, LI Liang2*, ZHANG Xiao1*. DNA Tetrahedral Nanostructure and its Application Progress in Biotechnology [J]. Curr. Biotech., 2020, 10(6): 661-667. |
| [10] | KE Zhenhua. Research and Development of Multiplex Real-time Fluorescence PCR Detection Method for Live Pathogenic Bacteria in Food and Beverage [J]. Curr. Biotech., 2020, 10(6): 717-727. |
| [11] | MA Xuan1, ZHANG Yangzi1, XU Wentao1,2*. Progress on Physicochemical Properties and Applications of Functional Nucleic Acid DNA Hydrogels [J]. Curr. Biotech., 2019, 9(6): 545-553. |
| [12] | DU Zaihui1, LUO Yunbo1, ZHU Longjiao1, XU Wentao1,2*. Special Secondary Structures of DNA and Their Application Progress [J]. Curr. Biotech., 2019, 9(6): 563-570. |
| [13] | LI Kai1,2, LUO Yunbo1,2, XU Wentao1,2*. Research Progress on CRISPR-Cas Mediated Biosensors [J]. Curr. Biotech., 2019, 9(6): 579-591. |
| [14] | ZHANG Yukun1,2, AN Na2, LIU Weixiao2, WAN Yusong2, JIN Wujun2, LI Liang2*, ZHANG Xiao1*. Research Progress of DNA Sensors Based on Surface Plasmon Resonance and Electrochemical Combination [J]. Curr. Biotech., 2019, 9(6): 592-598. |
| [15] | LIN Shenghao1, DU Zaihui1, ZHANG Xiujie2, HUANG Kunlun1,3, LIU Qingliang4, XU Wentao1,3*. Progress on Biosensors Based on Loop-mediated Isothermal Amplification [J]. Curr. Biotech., 2019, 9(6): 599-610. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||