Current Biotechnology ›› 2025, Vol. 15 ›› Issue (2): 226-233.DOI: 10.19586/j.2095-2341.2025.0011
• Reviews • Previous Articles Next Articles
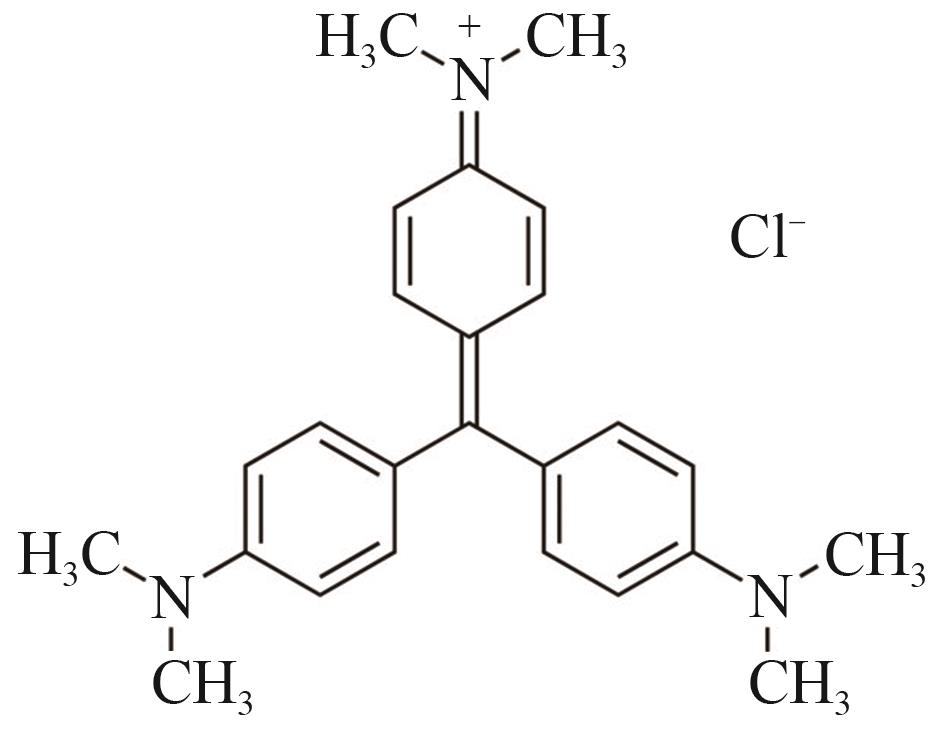
Anti-tumor Effects of Gentian Violet
- College of Basic Medicine,Inner Mongolia Medical University,Hohhot 010110,China
-
Received:2025-02-11Accepted:2025-02-28Online:2025-03-25Published:2025-04-29 -
Contact:Jianqiang WU
结晶紫的抗肿瘤作用
- 内蒙古医科大学基础医学院,呼和浩特 010110
-
通讯作者:武建强 -
作者简介:乌格叶木日 E-mail: 15947057516@163.com; -
基金资助:内蒙古自治区科技厅项目(2019GG037)
CLC Number:
Cite this article
Geyemuri WU, Jianqiang WU. Anti-tumor Effects of Gentian Violet[J]. Current Biotechnology, 2025, 15(2): 226-233.
乌格叶木日, 武建强. 结晶紫的抗肿瘤作用[J]. 生物技术进展, 2025, 15(2): 226-233.
share this article
| 1 | FATMA H, SIDDIQUE H R. Research and patents status of selected phytochemicals against cancer: how close and how far?[J]. Recent Pat. Anticancer Drug Discov., 2023, 18(4): 428-447. |
| 2 | DIORI KARIDIO I, SANLIER S H. Reviewing cancer's biology: an eclectic approach[J/OL]. J. Egypt. Natl. Canc. Inst., 2021, 33(1): 32[2025-01-02]. . |
| 3 | WU J, WOOD G S. Analysis of the effect of gentian violet on apoptosis and proliferation in cutaneous T-cell lymphoma in an in vitro study[J]. JAMA Dermatol., 2018, 154(10): 1191-1198. |
| 4 | CHEN J, ZHAO F, YANG H, et al.. Gentian violet induces apoptosis and ferroptosis via modulating p53 and MDM2 in hepatocellular carcinoma[J]. Am. J. Cancer Res., 2022, 12(7): 3357-3372. |
| 5 | PIETROBONO S, MORANDI A, GAGLIARDI S, et al.. Down-regulation of SOX2 underlies the inhibitory effects of the triphenylmethane gentian violet on melanoma cell self-renewal and survival[J]. J. Invest. Dermatol., 2016, 136(10): 2059-2069. |
| 6 | YAMAGUCHI M, VIKULINA T, WEITZMANN M N. Gentian violet inhibits MDA-MB-231 human breast cancer cell proliferation, and reverses the stimulation of osteoclastogenesis and suppression of osteoblast activity induced by cancer cells[J]. Oncol. Rep., 2015, 34(4): 2156-2162. |
| 7 | CHOI M S, KIM J H, LEE C Y, et al.. Gentian violet inhibits cell proliferation through induction of apoptosis in ovarian cancer cells[J/OL]. Biomedicines, 2023, 11(6): 1657[2025-01-02]. . |
| 8 | GARUFI A, D'ORAZI V, ARBISER J L, et al.. Gentian violet induces wtp53 transactivation in cancer cells[J]. Int. J. Oncol., 2014, 44(4): 1084-1090. |
| 9 | WESTERGAARD S A, LECHOWICZ M J, HARRINGTON M, et al.. Induction of remission in a patient with end-stage cutaneous T-cell lymphoma by concurrent use of radiation therapy, gentian violet, and mogamulizumab[J]. JAAD Case Rep., 2020, 6(8): 761-765. |
| 10 | ARBISER J L. Gentian violet is safe[J/OL]. J. Am. Acad. Dermatol., 2009, 61(2): 359[2025-01-02]. . |
| 11 | PRABHA N, ARORA R D, GANGULY S, et al.. Gentian violet: revisited[J]. Indian J. Dermatol. Venereol. Leprol., 2020, 86(5): 600-603. |
| 12 | DHAKAL S, BAGALE G, NEPAL A, et al.. Comparison of recovery rate of otomycosis using one-percent gentian violet and one-percent clotrimazole topical treatment[J]. J. Nepal Health Res. Counc., 2024, 22(2): 269-273. |
| 13 | O'TOOLE G A. Classic spotlight: how the gram stain works[J/OL]. J. Bacteriol., 2016, 198(23): 3128[2025-01-02]. . |
| 14 | DOCAMPO R, MORENO S N. The metabolism and mode of action of gentian violet[J]. Drug Metab. Rev., 1990, 22(2-3): 161-178. |
| 15 | BERRIOS R L, ARBISER J L. Effectiveness of gentian violet and similar products commonly used to treat pyodermas[J]. Dermatol. Clin., 2011, 29(1): 69-73. |
| 16 | FARID K J, KELLY K, ROSHIN S. Gentian violet 1% solution in the treatment of wounds in the geriatric patient: a retrospective study[J]. Geriatr. Nurs., 2011, 32(2): 85-95. |
| 17 | MALEY A M, ARBISER J L. Gentian violet: a 19th century drug re-emerges in the 21st century[J]. Exp. Dermatol., 2013, 22(12): 775-780. |
| 18 | TITFORD M. George grubler and karl hollborn: two founders of the biological stain industry[J]. J. Histotechnol., 1993, 16(2): 155-158. |
| 19 | BALABANOVA M, POPOVA L, TCHIPEVA R. Dyes in dermatology [J]. Clin. Dermatol., 2003, 21(1): 2-6. |
| 20 | CHURCHMAN J W. The selective bactericidal action of gentian violet[J]. J. Exp. Med., 1912, 16(2): 221-247. |
| 21 | HINTON D. Results of the intravenous use of gentian violet in cases of extreme septicaemia[J]. Ann. Surg., 1925, 81(3): 687-692. |
| 22 | SEBALD H A. Vincent's infection of the mouth[J]. Dent. Surv., 1948, 24: 45-47. |
| 23 | UTTER A R. Gentian violet treatment for thrush: can its use cause breastfeeding problems?[J]. J. Hum. Lact., 1990, 6(4): 178-180. |
| 24 | BUMBALO T S, GUSTINA F J. The treatment of pinworm infection (enterobiasis) with gentian violet suspension[J]. J. Pediatr., 1955, 47(3): 311-314. |
| 25 | MILLER M J, CHOQUETTE L, AUDET W, et al.. Studies on pinworm infection: 2. tests with gentian violet in the treatment of pinworm infection[J]. Can. Med. Assoc. J., 1940, 43(5): 455-458. |
| 26 | THOMPSON C W. Topical application of penicillin: solution, gentian-violet and heat in the treatment of extensive 1st and 2nd degree burns in children[J]. J. Natl. Med. Assoc., 1946, 38(1): 11-14. |
| 27 | WARNECKE W. Treatment of enterobiasis Vermicularis with gentian violet[J]. Med. Klin., 1950, 45(3): 737-739. |
| 28 | WRIGHT S C, MAREE J E, SIBANYONI M. Treatment of oral thrush in HIV/AIDS patients with lemon juice and lemon grass (Cymbopogon citratus) and gentian violet[J]. Phytomedicine, 2009, 16(2-3): 118-124. |
| 29 | ARBISER J L. Gentian violet: bench-to-bedside research that lowers healthcare costs[J]. Skinmed, 2016, 14(2): 91-92. |
| 30 | LIU J, TANG N, LIU N, et al.. Echinacoside inhibits the proliferation, migration, invasion and angiogenesis of ovarian cancer cells through PI3K/AKT pathway[J]. J. Mol. Histol., 2022, 53(2): 493-502. |
| 31 | LIN R, PIAO M, SONG Y, et al.. Quercetin suppresses AOM/DSS-induced colon carcinogenesis through its anti-inflammation effects in mice[J/OL]. J. Immunol. Res., 2020, 2020: 9242601[2025-01-02]. . |
| 32 | BHANDARKAR S S, MACKELFRESH J, FRIED L, et al.. Targeted therapy of oral hairy leukoplakia with gentian violet[J]. J. Am. Acad. Dermatol., 2008, 58(4): 711-712. |
| 33 | COOI L, WATANABE N, FUTAMURA Y, et al.. Identification of small molecule inhibitors of p27(Kip1) ubiquitination by high-throughput screening[J]. Cancer Sci., 2013, 104(11): 1461-1467. |
| 34 | DOBOS G, MILADI M, MICHEL L, et al.. Recent advances on cutaneous lymphoma epidemiology[J/OL]. Presse Med., 2022, 51(1): 104108[2025-01-02]. . |
| 35 | GOEL R R, ROOK A H. Immunobiology and treatment of cutaneous T-cell lymphoma[J]. Expert Rev. Clin. Immunol., 2024, 20(8): 985-996. |
| 36 | RAO S, MORRIS R, RICE Z P, et al.. Regression of diffuse B-cell lymphoma of the leg with intralesional gentian violet[J]. Exp. Dermatol., 2018, 27(1): 93-95. |
| 37 | 王姣姣,耿圆圆,张家耀,等.肝细胞癌循环肿瘤细胞标志物及检测方法研究进展[J].生物技术进展,2015,5(2):113-119. |
| WANG J J, GENG Y Y, ZHANG J Y, et al.. Progress for biomarkers and detection methods of circulating tumor cell of hepatocellular carcinoma[J]. Curr. Biotechnol., 2015, 5(2): 113-119. | |
| 38 | ARBISER J L, BIPS M, SEIDLER A, et al.. Combination therapy of imiquimod and gentian violet for cutaneous melanoma metastases[J]. J. Am. Acad. Dermatol., 2012, 67(2): e81-3. |
| 39 | 张鹏晓,胡念.黑色素瘤免疫治疗作用机制研究进展[J].生物技术进展,2023,13(6):900-906. |
| ZHANG P X, HU N. The research progress on action mechanism of melanoma immunotherapy[J]. Curr. Biotechnol., 2023, 13(6): 900-906. | |
| 40 | 赵轩,任丽梅,王晓茹,等.siRNA靶向干扰TRAF6的表达对肺癌细胞增殖与凋亡的影响[J].生物技术进展,2024,14(5):875-881. |
| ZHAO X, REN L M, WANG X R, et al.. Effects of siRNA targeting to interfere with the expression of TRAF6 on the proliferation and apoptosis of lung cancer cells[J]. Curr. Biotechnol., 2024, 14(5): 875-881. | |
| 41 | LONG G V, SWETTER S M, MENZIES A M, et al.. Cutaneous melanoma[J]. Lancet, 2023, 402(10400): 485-502. |
| 42 | RIBEIRO M B A J, DOS SANTOS G I, EVANGELISTA DOS S A C, et al.. Molecular landscape of hereditary melanoma[J/OL]. Crit. Rev. Oncol. Hematol., 2021, 164: 103425[2025-01-02]. . |
| 43 | ZHANG X, LIU R, YUAN Q, et al.. The precise diagnosis of cancer invasion/metastasis via 2D laser ablation mass mapping of metalloproteinase in primary cancer tissue[J]. ACS Nano, 2018, 12(11): 11139-11151. |
| 44 | 车逸宁, 李冕, 王晓, 等. ITGA7基因与人类肿瘤的泛癌分析 [J]. 生物技术进展, 2025, 15(1): 142-151. |
| CHE YN, LI M, WANG X, et al.. Pan cancer analysis between ITGA7 gene and human tumors[J]. Curr. Biotechnol., 2025, 15(1): 142-151. | |
| 45 | WILKINSON L, GATHANI T. Understanding breast cancer as a global health concern[J/OL]. Br. J. Radiol., 2022, 95(1130): 20211033[2025-01-02]. . |
| 46 | 孙莉莉,安外尔·约麦尔阿卜拉,刘富中,等.基于肿瘤相关成纤维细胞基因构建乳腺癌预后预测模型及免疫浸润分析[J].生物技术进展,2024,14(2):312-322. |
| SUN L L, ANWAIER Y, LIU F, , et al.. Construction of prognostic prediction model of breast cancer based on tumor-associated fibroblast genes and analysis of immune infiltration[J]. Curr. Biotechnol., 2024, 14(2): 312-322. | |
| 47 | YAMAGUCHI M, MURATA T. Potential suppressive effects of gentian violet on human breast cancer MDA-MB-231 cells in vitro: Comparison with gemcitabine[J]. Oncol. Lett., 2016, 12(2): 1605-1609. |
| 48 | MUKAWERA E, CHARTIER S, WILLIAMS V, et al.. Redox-modulating agents target NOX2-dependent IKKε oncogenic kinase expression and proliferation in human breast cancer cell lines[J]. Redox Biol., 2015, 6: 9-18. |
| 49 | 杜琳琳,谢飞,马雪梅.SALL4的促癌功能及治疗意义[J].生物技术进展,2023,13(5):704-711. |
| DU L L, XIE F, MA X M. Pro-oncogenic function and therapeutic significance of SALL4[J]. Curr. Biotechnol., 2023, 13(5): 704-711. | |
| 50 | CHANDRA A, PIUS C, NABEEL M, et al.. Ovarian cancer: current status and strategies for improving therapeutic outcomes[J]. Cancer Med., 2019, 8(16): 7018-7031. |
| 51 | BAIDOUN F, ELSHIWY K, ELKERAIE Y, et al.. Colorectal cancer epidemiology: recent trends and impact on outcomes[J]. Curr. Drug Targets, 2021, 22(9): 998-1009. |
| 52 | 杨梦恬,袁菊懋.RTN4对于结肠癌细胞增殖的调控作用[J].生物技术进展,2021,11(2):238-243. |
| YANG M T, YUAN J M. The regulation effect of RTN4 on colon cancer cell proliferation[J]. Curr. Biotechnol., 2021, 11(2): 238-243. | |
| 53 | ADZAVON Y M, 刘梦昱, 王惠, 等. 替莫唑胺治疗胶质母细胞瘤的耐药性产生机制研究进展 [J]. 生物技术进展, 2021, 11(6): 705-710. |
| ADZAVON Y M, LIU M Y, WANG H, et al.. Research progress of temozolomide in the mechanism of drug resistance in glioblastoma[J]. Curr. Biotechnol., 2021, 11(6): 705-710. | |
| 54 | 成于思, 胡钧涛, 胡胜利,等. CRISPR/Cas9敲除pyk2基因对人脑胶质瘤细胞增殖、迁移及侵袭能力的影响 [J]. 生物技术进展, 2017, 7(4): 338-344. |
| CHENG Y S, HU J T, HU S L, et al.. Effects of pyk2 knockout on the proliferation, migration and invasion of human glioma cell line by CRISPR/Cas9[J]. Curr. Biotechnol., 2017, 7(4): 338-344. | |
| 55 | KLEIN A P. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors[J]. Nat. Rev. Gastroenterol. Hepatol., 2021, 18(7): 493-502. |
| 56 | THAI A A, SOLOMON B J, SEQUIST L V, et al.. Lung cancer[J]. Lancet, 2021, 398(10299): 535-554. |
| 57 | 房俊伟, 冯添顺, 洪伟煊, 等. 基于衰老相关基因特征的胰腺癌风险分层及预后预测模型 [J]. 生物技术进展, 2025, 15(1): 158-169. |
| FANG J W, FENG T S, HONG W X, et al.. Pancreatic cancer risk stratification and prognostic prediction model based on aging-related gene characteristics[J]. Curr. Biotechnol., 2025, 15(1): 158-169. | |
| 58 | WANG Y Y, XIAO L Y, WU P C, et al.. Orabase-formulated gentian violet effectively improved oral potentially malignant disorder in vitro and in vivo [J/OL]. Biochem. Pharmacol., 2020, 171: 113713[2025-01-02]. . |
| 59 | SORS A, JEAN-LOUIS F, PELLET C, et al.. Down-regulating constitutive activation of the NF-kappaB canonical pathway overcomes the resistance of cutaneous T-cell lymphoma to apoptosis[J]. Blood, 2006, 107(6): 2354-2363. |
| 60 | SORS A, JEAN-LOUIS F, BÉGUÉ E, et al.. Inhibition of IkappaB kinase subunit 2 in cutaneous T-cell lymphoma down-regulates nuclear factor-kappaB constitutive activation, induces cell death, and potentiates the apoptotic response to antineoplastic chemotherapeutic agents[J]. Clin. Cancer Res., 2008, 14(3): 901-911. |
| 61 | CLEERE R, LONG A, KELLEHER D, et al.. Autocrine regulation of the transcription factor NF kappa B by TNF alpha in the human T cell lymphoma line Hut 78[J/OL]. Biochem. Soc. Trans., 1995, 23(1): 113S[2025-01-02]. . |
| 62 | O'CONNELL M A, CLEERE R, LONG A, et al.. Cellular proliferation and activation of NF kappa B are induced by autocrine production of tumor necrosis factor alpha in the human T lymphoma line HuT 78[J]. J. Biol. Chem., 1995, 270(13): 7399-7404. |
| 63 | IZBAN K F, ERGIN M, QIN J Z, et al.. Constitutive expression of NF-kappa B is a characteristic feature of mycosis fungoides: implications for apoptosis resistance and pathogenesis[J]. Hum. Pathol., 2000, 31(12): 1482-1490. |
| 64 | GIRI D K, AGGARWAL B B. Constitutive activation of NF-kappaB causes resistance to apoptosis in human cutaneous T cell lymphoma HuT-78 cells. Autocrine role of tumor necrosis factor and reactive oxygen intermediates[J]. J. Biol. Chem., 1998, 273(22): 14008-14014. |
| 65 | LI J, CAO F, YIN H L, et al.. Ferroptosis: past, present and future[J/OL]. Cell Death Dis., 2020, 11(2): 88[2025-01-02]. . |
| 66 | DONADEL G, GARZELLI C, FRANK R, et al.. Identification of a novel nuclear protein synthesized in growth-arrested human hepatoblastoma HepG2 cells[J]. Eur. J. Biochem., 1991, 195(3): 723-729. |
| 67 | GABRIELLI F, DONADEL G, BENSI G, et al.. A nuclear protein, synthesized in growth-arrested human hepatoblastoma cells, is a novel member of the short-chain alcohol dehydrogenase family[J]. Eur. J. Biochem., 1995, 232(2): 473-477. |
| 68 | DEISENROTH C, THORNER A R, ENOMOTO T, et al.. Mitochondrial Hep27 is a c-Myb target gene that inhibits Mdm2 and stabilizes p53[J]. Mol. Cell. Biol., 2010, 30(16): 3981-3993. |
| 69 | YAMAGUCHI M, VIKULINA T, ARBISER J L, et al.. Suppression of NF-κB activation by gentian violet promotes osteoblastogenesis and suppresses osteoclastogenesis[J]. Curr. Mol. Med., 2014, 14(6): 783-792. |
| 70 | KOPP B, KHOURY L, AUDEBERT M. Validation of the γH2AX biomarker for genotoxicity assessment: a review[J]. Arch. Toxicol., 2019, 93(8): 2103-2114. |
| [1] | Lulu ZHAO, Tian HONG, Yiran HAO, Erning CHEN, Jingwen LI, Meihong DU. Research Progress of Immunomagnetic Separation Technology in Detection of Circulating Tumor Cell [J]. Current Biotechnology, 2025, 15(4): 606-614. |
| [2] | Lina ZHU, Zhiling SONG. Autophagy and Apoptosis: Interactions and Their Role in Disease [J]. Current Biotechnology, 2025, 15(4): 622-626. |
| [3] | Xiaoyi ZHAI, Haiyue ZHANG, Wenjia GUO, Xiaogang DONG. Research Progress of Cancer-associated Fibroblasts in Breast Cancer [J]. Current Biotechnology, 2025, 15(4): 636-644. |
| [4] | Xiaoya LIU, Shuomin ZHANG, Peng ZHENG, Rui MA, Chaojun ZHANG. Role and Mechanisms of DBNDD1 in Colorectal Cancer Development [J]. Current Biotechnology, 2025, 15(4): 726-734. |
| [5] | Kaijing SUN, Xinze LIU, Xin JIN, Xue YANG, Qi WANG, Yu LI, Changbao CHEN, Xilin WAN. Research Progress on Antitumor Active Constituents and Their Mechanism of Action of Traditional Chinese Medicine Sanghuangporus baumii [J]. Current Biotechnology, 2024, 14(6): 929-936. |
| [6] | Beibei LI, Jianqiang WU. Research Progress of Fas-mediated Non-apoptotic Signaling in Tumor Cells [J]. Current Biotechnology, 2024, 14(3): 406-412. |
| [7] | Yan ZENG, Hengcheng ZHU, Kang YANG. The Mechanism of DNASE1L3 in Renal Cell Carcinoma [J]. Current Biotechnology, 2024, 14(3): 486-491. |
| [8] | Yeerkenbieke BUERLAN, Wenjia GUO, Xiaogang DONG. Advances on the Function of POSTN in Tumor Microenvironment [J]. Current Biotechnology, 2024, 14(2): 205-210. |
| [9] | Lili SUN, Yuemaierabola ANWAIER, Fuzhong LIU, Yeerkenbieke BUERLAN, Ye DILINAER, Wenjia GUO. Construction of Prognostic Prediction Model of Breast Cancer Based on Tumor-associated Fibroblast Genes and Analysis of Immune Infiltration [J]. Current Biotechnology, 2024, 14(2): 312-322. |
| [10] | Pengxiao ZHANG, Nian HU. The Research Progress on Action Mechanism of Melanoma Immunotherapy [J]. Current Biotechnology, 2023, 13(6): 900-906. |
| [11] | Yuemaierabola ANWAIER, Lili SUN, Yeerkenbieke BUERLAN, Wenjia GUO. Advances on the Role of Piezo1 in Cancer [J]. Current Biotechnology, 2023, 13(5): 712-717. |
| [12] | Wenbo MA, Yiqun PAN, Qun WANG, Zhuang MA, Minglian WANG, Yishu YANG. Preliminary Study on the Application of Graphene-coated Iron Nitride Magnetic Beads to Capture Lung Cancer Circulating Tumor Cells [J]. Current Biotechnology, 2023, 13(4): 628-636. |
| [13] | Yongchao LI, Zhao YANG. Status and Countermeasures of Bispecific Antibody Drugs [J]. Current Biotechnology, 2023, 13(3): 353-358. |
| [14] | Ying TANG, Jianqiang WU. Anti-tumor Effects of Phenylethanoid Glycosides Deprived from Cistanche deserticola [J]. Current Biotechnology, 2023, 13(3): 399-405. |
| [15] | Liqiang DONG, Bin WANG, Shi SU, Dongqi LIU. Progress on Celastrol in Anti-tumor Effects and Mechanism [J]. Current Biotechnology, 2023, 13(1): 77-82. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||