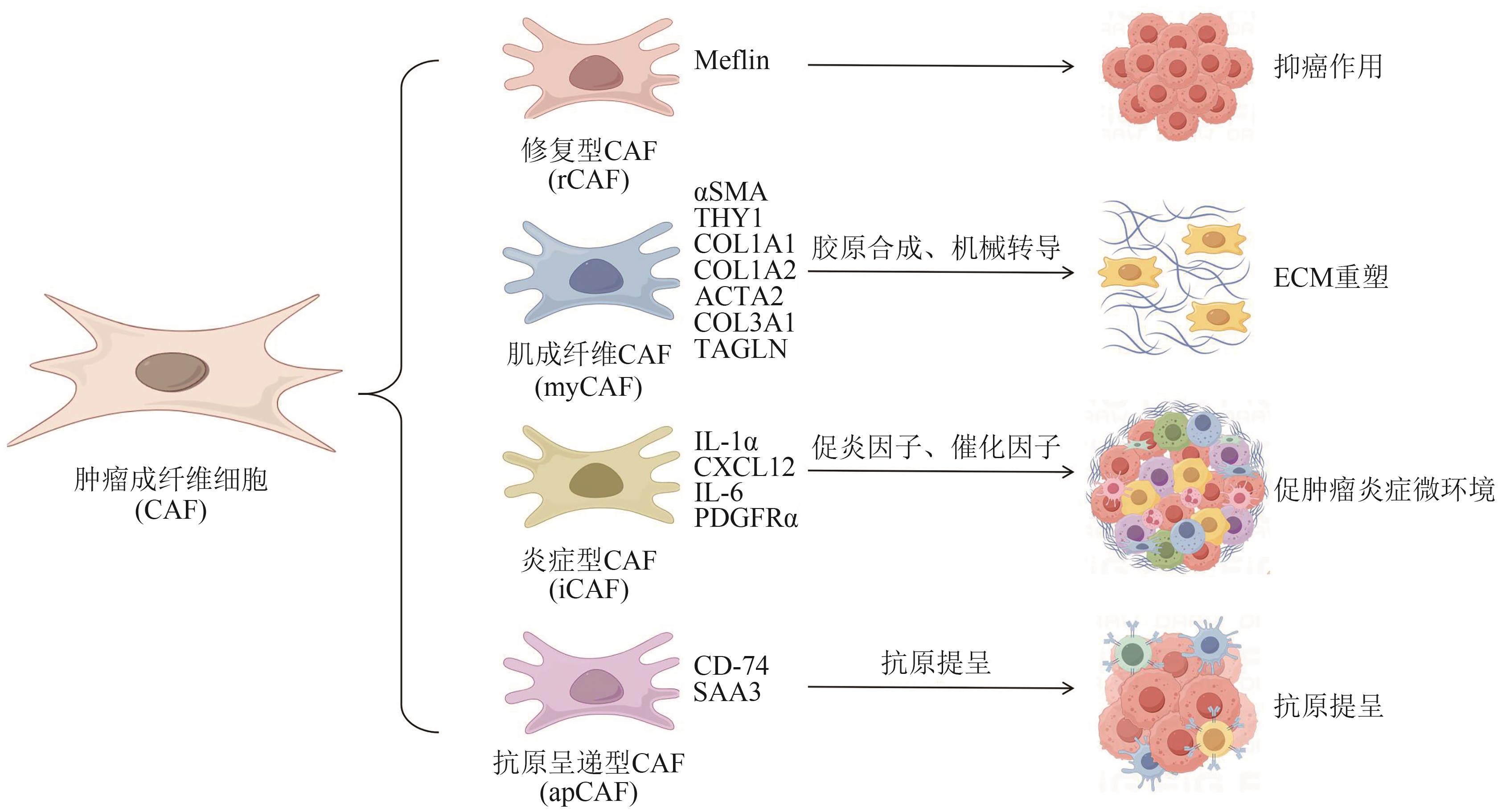
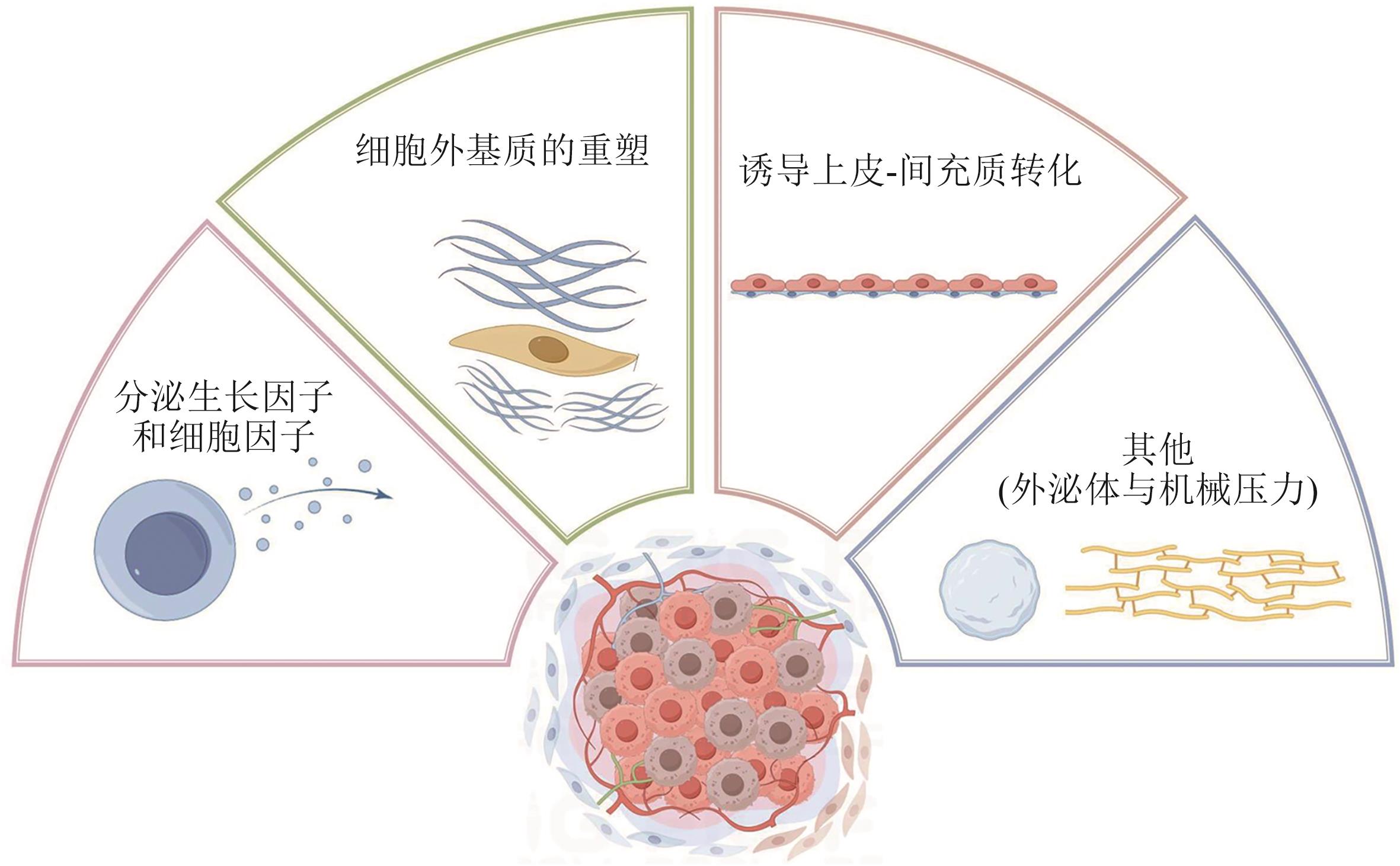
Current Biotechnology ›› 2025, Vol. 15 ›› Issue (4): 636-644.DOI: 10.19586/j.2095-2341.2024.0200
• Reviews • Previous Articles Next Articles
Research Progress of Cancer-associated Fibroblasts in Breast Cancer
Xiaoyi ZHAI1( ), Haiyue ZHANG1, Wenjia GUO1, Xiaogang DONG2(
), Haiyue ZHANG1, Wenjia GUO1, Xiaogang DONG2( )
)
- 1.Departments of Cancer Research Institute,Affiliated Cancer Hospital of Xinjiang Medical University,Urumqi 830011,China
2.Department of Hepatobiliary and Pancreatic Surgery,Cancer Hospital of Xinjiang Medical University,Urumqi 830011,China
-
Received:2024-12-18Accepted:2025-04-30Online:2025-07-25Published:2025-09-08 -
Contact:Xiaogang DONG
癌相关成纤维细胞在乳腺癌中的研究进展
- 1.新疆医科大学附属肿瘤医院肿瘤防治研究所,乌鲁木齐 830011
2.新疆医科大学附属肿瘤医院肝胆胰外科,乌鲁木齐 830011
-
通讯作者:董晓刚 -
作者简介:翟晓艺 E-mail:zhaixiaoyi0130@163.com; -
基金资助:新疆维吾尔自治区重点研发任务专项(2022B03019-4);2025年自治区高校基本科研业务费科研项目(XJEDU2025J059)
CLC Number:
Cite this article
Xiaoyi ZHAI, Haiyue ZHANG, Wenjia GUO, Xiaogang DONG. Research Progress of Cancer-associated Fibroblasts in Breast Cancer[J]. Current Biotechnology, 2025, 15(4): 636-644.
翟晓艺, 张海月, 郭文佳, 董晓刚. 癌相关成纤维细胞在乳腺癌中的研究进展[J]. 生物技术进展, 2025, 15(4): 636-644.
share this article
| 生物标志物 | 定位 | CAFs中的表达水平 | 描述 | 生物学作用 |
|---|---|---|---|---|
| 波形蛋白 (vimentin) | 细胞质 | ↑ | Ⅲ型中间拉丝 | 肿瘤生长、浸润和迁移 |
| α-平滑肌肌动蛋白 (α-SMA) | 细胞质 | ↓ | 与细胞收缩、运动、结构和完整性有关 | 肿瘤生长,药物屏障,ECM重塑 |
| 人成纤维细胞特异蛋白1 (FSP1) | 细胞质 | ↑ | 与细胞运动、胶原诱导和组织纤维化有关 | 免疫逃避,免疫监视,纤维化 |
| 成纤维细胞激活蛋白 (FAP) | 膜 | ↑ | 与纤维生成和ECM重塑有关 | 肿瘤的进展和转移,塑造免疫微环境,ECM重塑,纤维形成 |
| 人腱糖蛋白C (tenascin-C) | ECM蛋白 | ↑ | 与细胞黏附有关的ECM糖蛋白 | 纤维化,EMT |
| 结蛋白 (desmin) | 细胞质 | ↓ | Ⅲ型中间拉丝 | 细胞黏附,细胞迁移 |
| 血小板衍生生长因子受体α/β (PDGFRα/β) | 膜 | ↑ | 酪氨酸激酶受体 | M2极化,血管形成 |
| 窖蛋白 (caveolin-1) | 膜 | ↑;↓ | 支架蛋白 | 癌细胞转移 |
| 平足蛋白 (p | 膜 | ↑ | Ⅰ型整体膜糖蛋白 | 免疫抑制,肿瘤生长 |
Table 1 Biomaker of CAFs
| 生物标志物 | 定位 | CAFs中的表达水平 | 描述 | 生物学作用 |
|---|---|---|---|---|
| 波形蛋白 (vimentin) | 细胞质 | ↑ | Ⅲ型中间拉丝 | 肿瘤生长、浸润和迁移 |
| α-平滑肌肌动蛋白 (α-SMA) | 细胞质 | ↓ | 与细胞收缩、运动、结构和完整性有关 | 肿瘤生长,药物屏障,ECM重塑 |
| 人成纤维细胞特异蛋白1 (FSP1) | 细胞质 | ↑ | 与细胞运动、胶原诱导和组织纤维化有关 | 免疫逃避,免疫监视,纤维化 |
| 成纤维细胞激活蛋白 (FAP) | 膜 | ↑ | 与纤维生成和ECM重塑有关 | 肿瘤的进展和转移,塑造免疫微环境,ECM重塑,纤维形成 |
| 人腱糖蛋白C (tenascin-C) | ECM蛋白 | ↑ | 与细胞黏附有关的ECM糖蛋白 | 纤维化,EMT |
| 结蛋白 (desmin) | 细胞质 | ↓ | Ⅲ型中间拉丝 | 细胞黏附,细胞迁移 |
| 血小板衍生生长因子受体α/β (PDGFRα/β) | 膜 | ↑ | 酪氨酸激酶受体 | M2极化,血管形成 |
| 窖蛋白 (caveolin-1) | 膜 | ↑;↓ | 支架蛋白 | 癌细胞转移 |
| 平足蛋白 (p | 膜 | ↑ | Ⅰ型整体膜糖蛋白 | 免疫抑制,肿瘤生长 |
| 免疫细胞类型 | 与CAFs的关系 | 研究结果 | 参考文献 |
|---|---|---|---|
| 巨噬细胞(tumor-associated macrophages,TAMs) | CAFs通过分泌单核细胞趋化蛋白-1(cc chemokine ligand 2,CCL2)等因子吸引单核细胞进入肿瘤,并促使其极化为M2型巨噬细胞,这种巨噬细胞具有免疫抑制特性,能够促进肿瘤生长和侵袭。研究表明,高密度的CAFs与更多的CD163或CD206阳性巨噬细胞在乳腺癌组织中密切相关。CAFs通过分泌IL-6、粒细胞-巨噬细胞集落刺激因子等分子招募并极化TAMs为M2型,形成一个促进肿瘤生长的免疫抑制环境 | CAFs能够诱导M1型巨噬细胞向M2型转化,增强免疫抑制作用。CAFs通过分泌细胞因子(如MCP-1、SDF-1等)招募单核细胞,并促进其向M2型巨噬细胞分化 | [ |
| 自然杀伤细胞(natural killer cells,NK) | CAFs分泌金属蛋白酶(matrix metalloproteinases, MMPs)减少NK细胞表面激活受体(如NKG2D配体)的表达,削弱NK细胞的毒性。此外,CAFs通过分泌吲哚胺2,3-二氧酶或前列腺素E2(PGE2),降低NK细胞的活性。CAFs通过分泌TGF-β、PGE2等分子抑制NK细胞的活性,降低其表面的NKG2D等受体的表达,削弱其对肿瘤细胞的杀伤能力。此外,CAFs分泌的MMPs减少了肿瘤细胞表面的MHCI类链相关分子,进一步降低了NK细胞的杀伤效率 | CAFs通过分泌前列腺素E2和TGF-β抑制NK细胞的活性,降低其对肿瘤细胞的杀伤能力。且CAFs的细胞表面表达降低了NK细胞激活受体的表达,进一步影响NK细胞的抗肿瘤活性 | [ |
| 树突状细胞(dendritic cells,DCs) | CAFs分泌的TGF-β和IL-6等因子抑制DCs的成熟和功能,减少抗原提呈能力,减弱DCs激发T细胞应答的效果。CAFs还能通过代谢色氨酸产生Kyn,抑制DC的分化和功能,促进肿瘤免疫 | 肝细胞癌来源的CAFs能够促进调节性DCs的生成,进一步抑制T细胞增殖 | [ |
| 肿瘤浸润性淋巴细胞(tumor-infiltrating lymphocytes,TILs) | 主要由CD4+和CD8+T细胞构成。CAFs通过分泌多种细胞因子调节这两种T细胞的功能。例如,CAFs分泌的胸腺基质淋巴生成素(thymic stromal lymphopoietin,TSLP)通过调节骨髓样树突状细胞,促进Th2极化,而在原发性肿瘤中使用FAP+CAFs DNA疫苗可以增加IL-2、IL-7等Th1细胞因子的表达,同时减少Th2细胞因子(如IL-4、IL-6),增强细胞毒性T淋巴细胞的杀伤力 | 当CAFs数量较多时,肿瘤内CD8+ TILs数量显著减少,而FOXp3+ TILs数量增加,表明CAFs可能通过调节TIL的迁移实现免疫抑制 | [ |
| 髓系来源抑制性细胞(myeloid-derived suppressor cells, MDSCs) | CAFs通过SDF-1α/CXCR4途径吸引单核细胞,并通过IL-6介导的STAT3激活诱导单核细胞分化为MDSCs。MDSCs通过产生活性氧(reactive oxygen species,ROS)、一氧化氮和免疫抑制因子(如IL-10),抑制CD8+ T细胞的活性,影响抗肿瘤免疫 | MDSCs能够抑制T细胞增殖,改变T细胞的表型和功能,导致肿瘤进展 | [ |
| 肥大细胞(mast cells,MCs) | MCs与CAFs相互作用,本文中MCs通过促进纤维细胞的重构支持肿瘤生长 | 抗肿瘤药物如Trinostat和Tranilast的应用揭示了MCs和CAFs对免疫抑制微环境形成的重要影响 | [ |
| 中性粒细胞(neutrophils) | CAFs通过CXCR2介导的化学趋化因子信号招募并极化TANs | CAFs通过抑制TANs的功能进一步促进肿瘤的侵袭性 | [ |
Table 2 Relationship between CAFs and immune cells
| 免疫细胞类型 | 与CAFs的关系 | 研究结果 | 参考文献 |
|---|---|---|---|
| 巨噬细胞(tumor-associated macrophages,TAMs) | CAFs通过分泌单核细胞趋化蛋白-1(cc chemokine ligand 2,CCL2)等因子吸引单核细胞进入肿瘤,并促使其极化为M2型巨噬细胞,这种巨噬细胞具有免疫抑制特性,能够促进肿瘤生长和侵袭。研究表明,高密度的CAFs与更多的CD163或CD206阳性巨噬细胞在乳腺癌组织中密切相关。CAFs通过分泌IL-6、粒细胞-巨噬细胞集落刺激因子等分子招募并极化TAMs为M2型,形成一个促进肿瘤生长的免疫抑制环境 | CAFs能够诱导M1型巨噬细胞向M2型转化,增强免疫抑制作用。CAFs通过分泌细胞因子(如MCP-1、SDF-1等)招募单核细胞,并促进其向M2型巨噬细胞分化 | [ |
| 自然杀伤细胞(natural killer cells,NK) | CAFs分泌金属蛋白酶(matrix metalloproteinases, MMPs)减少NK细胞表面激活受体(如NKG2D配体)的表达,削弱NK细胞的毒性。此外,CAFs通过分泌吲哚胺2,3-二氧酶或前列腺素E2(PGE2),降低NK细胞的活性。CAFs通过分泌TGF-β、PGE2等分子抑制NK细胞的活性,降低其表面的NKG2D等受体的表达,削弱其对肿瘤细胞的杀伤能力。此外,CAFs分泌的MMPs减少了肿瘤细胞表面的MHCI类链相关分子,进一步降低了NK细胞的杀伤效率 | CAFs通过分泌前列腺素E2和TGF-β抑制NK细胞的活性,降低其对肿瘤细胞的杀伤能力。且CAFs的细胞表面表达降低了NK细胞激活受体的表达,进一步影响NK细胞的抗肿瘤活性 | [ |
| 树突状细胞(dendritic cells,DCs) | CAFs分泌的TGF-β和IL-6等因子抑制DCs的成熟和功能,减少抗原提呈能力,减弱DCs激发T细胞应答的效果。CAFs还能通过代谢色氨酸产生Kyn,抑制DC的分化和功能,促进肿瘤免疫 | 肝细胞癌来源的CAFs能够促进调节性DCs的生成,进一步抑制T细胞增殖 | [ |
| 肿瘤浸润性淋巴细胞(tumor-infiltrating lymphocytes,TILs) | 主要由CD4+和CD8+T细胞构成。CAFs通过分泌多种细胞因子调节这两种T细胞的功能。例如,CAFs分泌的胸腺基质淋巴生成素(thymic stromal lymphopoietin,TSLP)通过调节骨髓样树突状细胞,促进Th2极化,而在原发性肿瘤中使用FAP+CAFs DNA疫苗可以增加IL-2、IL-7等Th1细胞因子的表达,同时减少Th2细胞因子(如IL-4、IL-6),增强细胞毒性T淋巴细胞的杀伤力 | 当CAFs数量较多时,肿瘤内CD8+ TILs数量显著减少,而FOXp3+ TILs数量增加,表明CAFs可能通过调节TIL的迁移实现免疫抑制 | [ |
| 髓系来源抑制性细胞(myeloid-derived suppressor cells, MDSCs) | CAFs通过SDF-1α/CXCR4途径吸引单核细胞,并通过IL-6介导的STAT3激活诱导单核细胞分化为MDSCs。MDSCs通过产生活性氧(reactive oxygen species,ROS)、一氧化氮和免疫抑制因子(如IL-10),抑制CD8+ T细胞的活性,影响抗肿瘤免疫 | MDSCs能够抑制T细胞增殖,改变T细胞的表型和功能,导致肿瘤进展 | [ |
| 肥大细胞(mast cells,MCs) | MCs与CAFs相互作用,本文中MCs通过促进纤维细胞的重构支持肿瘤生长 | 抗肿瘤药物如Trinostat和Tranilast的应用揭示了MCs和CAFs对免疫抑制微环境形成的重要影响 | [ |
| 中性粒细胞(neutrophils) | CAFs通过CXCR2介导的化学趋化因子信号招募并极化TANs | CAFs通过抑制TANs的功能进一步促进肿瘤的侵袭性 | [ |
| 药物 | 临床阶段 | 作用方式 |
|---|---|---|
| 吡非尼酮 | Ⅰ期 | 抑制增殖、下调TGFB、PDGF、胶原蛋白合成 |
| 氯沙坦 | 临床前 | 胶原蛋白Ⅰ合成抑制剂 |
| 多西他赛 | Ⅰb期 | 加强微管蛋白聚合作用和抑制微管解聚作用 |
| ProAgio | 临床前 | 整合素αυβ3,减少胶原蛋白,降低CAFs分泌蛋白 |
| 鲁索替尼 | Ⅱ期 | JAK/STAT通路和DNA甲基转移酶活性抑制剂 |
| 全反式维A酸 | Ⅰ期 | 通过MCL2下调激动肌球蛋白收缩力,重编程CAFs静止 |
| HA-PTX | 临床前 | MMP(抑制血管生成和ECM降解) |
| 青蒿素衍生物 | 临床前 | 抑制TGFβ释放 |
| 泛FGFR抑制剂 | 临床前 | 抑制CAFs激活 |
Table 3 Drugs targeting CAFs in breast cancer
| 药物 | 临床阶段 | 作用方式 |
|---|---|---|
| 吡非尼酮 | Ⅰ期 | 抑制增殖、下调TGFB、PDGF、胶原蛋白合成 |
| 氯沙坦 | 临床前 | 胶原蛋白Ⅰ合成抑制剂 |
| 多西他赛 | Ⅰb期 | 加强微管蛋白聚合作用和抑制微管解聚作用 |
| ProAgio | 临床前 | 整合素αυβ3,减少胶原蛋白,降低CAFs分泌蛋白 |
| 鲁索替尼 | Ⅱ期 | JAK/STAT通路和DNA甲基转移酶活性抑制剂 |
| 全反式维A酸 | Ⅰ期 | 通过MCL2下调激动肌球蛋白收缩力,重编程CAFs静止 |
| HA-PTX | 临床前 | MMP(抑制血管生成和ECM降解) |
| 青蒿素衍生物 | 临床前 | 抑制TGFβ释放 |
| 泛FGFR抑制剂 | 临床前 | 抑制CAFs激活 |
| [1] | ROHEEL A, KHAN A, ANWAR F, et al.. Global epidemiology of breast cancer based on risk factors: a systematic review[J/OL]. Front. Oncol., 2023, 13: 1240098[2025-03-10]. . |
| [2] | SUNG H, FERLAY J, SIEGEL R L, et al.. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J. Clin., 2021, 71(3): 209-249. |
| [3] | PEDERSEN R N, MELLEMKJÆR L, EJLERTSEN B, et al.. Mortality after late breast cancer recurrence in Denmark[J]. J. Clin. Oncol., 2022, 40(13): 1450-1463. |
| [4] | LIU J, YUAN Q, GUO H, et al.. Deciphering drug resistance in gastric cancer: potential mechanisms and future perspectives[J/OL]. Biomed. Pharmacother., 2024, 173: 116310[2025-03-10]. . |
| [5] | 孙莉莉,安外尔·约麦尔阿卜拉,刘富中,等.基于肿瘤相关成纤维细胞基因构建乳腺癌预后预测模型及免疫浸润分析[J].生物技术进展,2024,14(2):312-322. |
| SUN L L, ANWAIER Y, LIU F Z, et al.. Construction of prognostic prediction model of breast cancer based on tumor-associated fibroblast genes and analysis of immune infiltration[J]. Curr. Biotechnol., 2024, 14(2): 312-322. | |
| [6] | PIWOCKA O, PIOTROWSKI I, SUCHORSKA W M, et al.. Dynamic interactions in the tumor niche: how the cross-talk between CAFs and the tumor microenvironment impacts resistance to therapy[J/OL]. Front. Mol. Biosci., 2024, 11: 1343523[2025-03-10]. . |
| [7] | SØLAND T M, LIPKA A, KRUUS A, et al.. Extracellular vesicles from cancer cell lines of different origins drive the phenotype of normal oral fibroblasts in a CAF-like direction[J/OL]. Front. Oncol., 2024, 14: 1456346[2025-03-10]. . |
| [8] | PLIKUS M V, WANG X, SINHA S, et al.. Fibroblasts: origins, definitions, and functions in health and disease[J]. Cell, 2021, 184(15): 3852-3872. |
| [9] | YASUDA M, MIYACHI Y, ISHIKAWA O, et al.. Spatial expressions of fibronectin and integrins by human and rodent dermal fibroblasts[J]. Br. J. Dermatol., 2006, 155(3): 522-531. |
| [10] | KOBAYASHI T, YAMASHITA A, TSUMAKI N, et al.. Subpopulations of fibroblasts derived from human iPS cells[J/OL]. Commun. Biol., 2024, 7: 736[2025-03-10]. . |
| [11] | IRVINE A F, WAISE S, GREEN E W, et al.. Characterising cancer-associated fibroblast heterogeneity in non-small cell lung cancer: a systematic review and meta-analysis[J/OL]. Sci. Rep., 2021, 11: 3727[2025-03-10]. . |
| [12] | SHI Y, WU Z, LIU S, et al.. Targeting PRMT3 impairs methylation and oligomerization of HSP60 to boost anti-tumor immunity by activating cGAS/STING signaling[J/OL]. Nat. Commun., 2024, 15: 7930[2025-03-10]. . |
| [13] | ZHENG L, CAI W, KE Y, et al.. Cancer-associated fibroblasts: a pivotal regulator of tumor microenvironment in the context of radiotherapy[J/OL]. Cell Commun. Signal., 2025, 23(1): 147[2025-03-10]. . |
| [14] | LIU Y, SINJAB A, MIN J, et al.. Conserved spatial subtypes and cellular neighborhoods of cancer-associated fibroblasts revealed by single-cell spatial multi-omics[J]. Cancer Cell, 2025, 43(5): 905-924.e6. |
| [15] | NAITO K, SANGAI T, YAMASHITA K. CAF-associated genes in breast cancer for novel therapeutic strategies[J/OL]. Biomedicines, 2024, 12(9): 1964[2025-03-10]. . |
| [16] | MA C, YANG C, PENG A, et al.. Pan-cancer spatially resolved single-cell analysis reveals the crosstalk between cancer-associated fibroblasts and tumor microenvironment[J/OL]. Mol. Cancer, 2023, 22(1): 170[2025-03-10]. . |
| [17] | FANG Z, MENG Q, XU J, et al.. Signaling pathways in cancer-associated fibroblasts: recent advances and future perspectives[J]. Cancer Commun., 2023, 43(1): 3-41. |
| [18] | GLABMAN R A, CHOYKE P L, SATO N. Cancer-associated fibroblasts: tumorigenicity and targeting for cancer therapy[J/OL]. Cancers, 2022, 14(16): 3906[2025-03-10]. . |
| [19] | HU D, LI Z, ZHENG B, et al.. Cancer-associated fibroblasts in breast cancer: challenges and opportunities[J]. Cancer Commun., 2022, 42(5): 401-434. |
| [20] | CALIGIURI G, TUVESON D A. Activated fibroblasts in cancer: perspectives and challenges[J]. Cancer Cell, 2023, 41(3): 434-449. |
| [21] | CORDS L, TIETSCHER S, ANZENEDER T, et al.. Cancer-associated fibroblast classification in single-cell and spatial proteomics data[J/OL]. Nat. Commun., 2023, 14: 4294[2025-03-10]. . |
| [22] | SATO R, IMAMURA K, SEMBA T, et al.. TGFβ signaling activated by cancer-associated fibroblasts determines the histological signature of lung adenocarcinoma[J]. Cancer Res, 2021, 81(18): 4751-4765. |
| [23] | JIA H, CHEN X, ZHANG L, et al.. Cancer associated fibroblasts in cancer development and therapy[J/OL]. J. Hematol. Oncol., 2025, 18(1): 36[2025-03-10]. . |
| [24] | PRAKASH J, SHAKED Y. The interplay between extracellular matrix remodeling and cancer therapeutics[J]. Cancer Discov., 2024, 14(8): 1375-1388. |
| [25] | HUANG H, WANG Z, ZHANG Y, et al.. Mesothelial cell-derived antigen-presenting cancer-associated fibroblasts induce expansion of regulatory T cells in pancreatic cancer[J]. Cancer Cell, 2022, 40(6): 656-673. |
| [26] | RUISHI X, LINYI X, YUNFAN B, et al.. New perspectives on chemokines in hepatocellular carcinoma therapy: a critical pathway for natural products regulation of the tumor microenvironment[J/OL]. Front Immunol., 2024,15:1456405[2025-03-10]. . |
| [27] | GAO D, FANG L, LIU C, et al.. Microenvironmental regulation in tumor progression: interactions between cancer-associated fibroblasts and immune cells[J/OL]. Biomed. Pharmacother, 2023, 167: 115622[2025-03-10]. . |
| [28] | 慕广,张文豪,黄晶晶,等.肿瘤相关成纤维细胞对于免疫细胞的调节作用研究现状[J].中国肺癌杂志,2022,25(3):207-213. |
| MU G, ZHANG W H, HUANG J J, et al.. Research status of tumor-associated fibroblasts regulating immune cells[J]. Chin. J. Lung Cancer, 2022, 25(3): 207-213. | |
| [29] | DIEP C H, SPARTZ A, TRUONG T H, et al.. Progesterone receptor signaling promotes cancer associated fibroblast mediated tumorigenicity in ER+ breast cancer[J/OL]. Endocrinology, 2024, 165(9): bqae092[2025-03-10]. . |
| [30] | MARIN A, MORALES F, WALBAUM B. Fibroblast growth factor receptor signaling in estrogen receptor-positive breast cancer: mechanisms and role in endocrine resistance[J/OL]. Front. Oncol., 2024, 14: 1406951[2025-03-10]. . |
| [31] | NEDAEINIA R, NAJAFGHOLIAN S, SALEHI R, et al.. The role of cancer-associated fibroblasts and exosomal miRNAs-mediated intercellular communication in the tumor microenvironment and the biology of carcinogenesis: a systematic review[J/OL]. Cell Death Discov., 2024, 10: 380[2025-03-10]. . |
| [32] | ZHENG J H, ZHU Y H, YANG J, et al.. A CLIC1 network coordinates matrix stiffness and the Warburg effect to promote tumor growth in pancreatic cancer[J/OL]. Cell Rep., 2024, 43(8): 114633[2025-03-10]. . |
| [33] | XIE R, XU L, BAI Y. Progress of the study on type Ⅲ collagen in breast cancer[J]. Adv. Clin. Med., 2024, 14(04): 2295-304. |
| [34] | LIANG D, LIU L, ZHAO Y, et al.. Targeting extracellular matrix through phytochemicals: a promising approach of multi-step actions on the treatment and prevention of cancer[J/OL]. Front. Pharmacol., 2023, 14: 1186712[2025-03-10]. . |
| [35] | OZMEN E, DEMIR T D, OZCAN G. Cancer-associated fibroblasts: protagonists of the tumor microenvironment in gastric cancer[J/OL]. Front. Mol. Biosci., 2024, 11: 1340124[2025-03-10]. . |
| [36] | WANG Y, LYU W, YI Y, et al.. A novel signature based on cancer-associated fibroblast genes to predict prognosis, immune feature, and therapeutic response in breast cancer[J]. Aging, 2023, 15(9): 3480-3497. |
| [37] | WRIGHT K, LY T, KRIET M, et al.. Cancer-associated fibroblasts: master tumor microenvironment modifiers[J/OL]. Cancers, 2023, 15(6): 1899[2025-03-10]. . |
| [38] | LI J, WANG X, LIU R, et al.. Lysyl oxidase (LOX) family proteins: key players in breast cancer occurrence and progression[J]. J. Cancer, 2024, 15(16): 5230-5243. |
| [39] | FARALLI J A, FILLA M S, PETERS D M. Role of integrins in the development of fibrosis in the trabecular meshwork[J/OL]. Front. Ophthalmol., 2023, 3: 1274797[2025-03-10]. . |
| [40] | MGRDITCHIAN T, BROWN-CLAY J, HOFFMANN C, et al.. Actin cytoskeleton depolymerization increases matrix metalloproteinase gene expression in breast cancer cells by promoting translocation of cysteine-rich protein 2 to the nucleus[J/OL]. Front. Cell Dev. Biol., 2023, 11: 1100938[2025-03-10]. . |
| [41] | HOHMANN L, SIGURJONSDOTTIR K, CAMPOS A B, et al.. Genomic characterization of the HER2-enriched intrinsic molecular subtype in primary ER-positive HER2-negative breast cancer[J/OL]. Nat. Commun., 2025, 16: 2208[2025-03-10]. . |
| [42] | MOASSER M M. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis[J]. Oncogene, 2007, 26(45): 6469-6487. |
| [43] | 刘亚茹,马俊丽.肿瘤微环境在三阴性乳腺癌中的研究进展[J].临床医学进展,2023,13(7):11713-11720. |
| LIU Y R, MA J L. The progress of tumour microenvironment in triple-negative breast cancer[J]. Adv. Clin. Med., 2023, 13(7): 11713-11720. | |
| [44] | PÉREZ-GONZÁLEZ A, BÉVANT K, BLANPAIN C. Cancer cell plasticity during tumor progression, metastasis and response to therapy[J]. Nat. Cancer, 2023, 4(8): 1063-1082. |
| [45] | ZHANG Y, NADERI YEGANEH P, ZHANG H, et al.. Tumor editing suppresses innate and adaptive antitumor immunity and is reversed by inhibiting DNA methylation[J]. Nat. Immunol., 2024, 25(10): 1858-1870. |
| [46] | CHHABRA Y, WEERARATNA A T. Fibroblasts in cancer: unity in heterogeneity[J]. Cell, 2023, 186(8): 1580-1609. |
| [47] | ČERMÁKOVÁ K, ŠIMKOVÁ A, WICHTERLE F, et al.. Sensitive quantification of fibroblast activation protein and high-throughput screening for inhibition by FDA-approved compounds[J/OL]. Eur. J. Med. Chem., 2024, 280: 116948[2025-03-10]. . |
| [48] | JIANG Y, TIAN Y, FENG B, et al.. A novel molecular imaging probe [99mTc] Tc-HYNIC-FAPI targeting cancer-associated fibroblasts[J/OL]. Sci. Rep., 2023, 13: 3700[2025-03-10]. . |
| [49] | RUAN Q, WANG Q, JIANG Y, et al.. Synthesis and evaluation of 99mTc-labeled FAP inhibitors with different linkers for imaging of fibroblast activation proteins in tumors[J]. J. Med. Chem., 2023, 66(7): 4952-4960. |
| [50] | QIN S, GUO Q, LIU Y, et al.. A novel TGFbeta/TGILR axis mediates crosstalk between cancer-associated fibroblasts and tumor cells to drive gastric cancer progression[J/OL]. Cell Death Dis., 2024, 15(5): 368[2025-03-10]. . |
| [51] | DU F, LI J, ZHONG X, et al.. Endothelial-to-mesenchymal transition in the tumor microenvironment: roles of transforming growth factor-β and matrix metalloproteins[J/OL]. Heliyon, 2024, 10(21): e40118[2025-03-10]. . |
| [52] | CHANDRA J B, SARKAR S, ROUT L, et al.. The transformation of cancer-associated fibroblasts: current perspectives on the role of TGF-β in CAF mediated tumor progression and therapeutic resistance[J]. Cancer Lett., 2021, 520: 222-232. |
| [53] | CHEN M, CHEN F, GAO Z, et al.. CAFs and T cells interplay: the emergence of a new arena in cancer combat[J/OL]. Biomed. Pharmacother., 2024, 177: 117045[2025-03-10]. . |
| [54] | YIN H, SUN L, YUAN Y, et al.. PPIC-labeled CAFs: key players in neoadjuvant chemotherapy resistance for gastric cancer[J/OL]. Transl. Oncol., 2024, 48: 102080[2025-03-10]. . |
| [1] | Xiaoya LIU, Shuomin ZHANG, Peng ZHENG, Rui MA, Chaojun ZHANG. Role and Mechanisms of DBNDD1 in Colorectal Cancer Development [J]. Current Biotechnology, 2025, 15(4): 726-734. |
| [2] | Ziyi ZHANG-HUANG, Lisha HUANG, Yanqi LI, Chenlu XIONG, Ying YU, Fei XIE. Construction of a Breast Cancer Prognostic Model Based on Nicotine Metabolism Gene Signatures [J]. Current Biotechnology, 2025, 15(4): 735-742. |
| [3] | Yuqin BIAN, Keran DONG, Junzi LU, Enming ZHONG, Wanying GU, Jingshu ZHAO, Hongshu SUI. Clinical Progress in Targeted Therapy and Immunotherapy in Breast Cancer [J]. Current Biotechnology, 2025, 15(2): 234-240. |
| [4] | Yeerkenbieke BUERLAN, Lili SUN, Yuemaierabola ANWAIER, Wenjia GUO. Comprehensive Analysis of POSTN in ER+ Breast Cancer——Based on Single-cell RNA and Bulk RNA-seq Sequencing [J]. Current Biotechnology, 2024, 14(6): 1055-1066. |
| [5] | Yeerkenbieke BUERLAN, Wenjia GUO, Xiaogang DONG. Advances on the Function of POSTN in Tumor Microenvironment [J]. Current Biotechnology, 2024, 14(2): 205-210. |
| [6] | Lili SUN, Yuemaierabola ANWAIER, Fuzhong LIU, Yeerkenbieke BUERLAN, Ye DILINAER, Wenjia GUO. Construction of Prognostic Prediction Model of Breast Cancer Based on Tumor-associated Fibroblast Genes and Analysis of Immune Infiltration [J]. Current Biotechnology, 2024, 14(2): 312-322. |
| [7] | Yuemaierabola ANWAIER, Yeerkenbieke BUERLAN, Lili SUN, Fuzhong LIU, Yeerxiati DILINAER, Wenjia GUO. Prognosis Prediction Model and Drug Sensitivity Analysis of Triple-negative Breast Cancer Based on m5C Related Genes [J]. Current Biotechnology, 2024, 14(1): 149-159. |
| [8] | Pengxiao ZHANG, Nian HU. The Research Progress on Action Mechanism of Melanoma Immunotherapy [J]. Current Biotechnology, 2023, 13(6): 900-906. |
| [9] | Yongchao LI, Zhao YANG. Status and Countermeasures of Bispecific Antibody Drugs [J]. Current Biotechnology, 2023, 13(3): 353-358. |
| [10] | Xin HU, Huici MA, Mingsheng HAN, Xiaohong YUAN, Mingyu YANG, Yanqin MA. Screening of Triple⁃negative Breast Cancer⁃associated miRNAs and Bioinfor⁃matics Analysis of the Target Genes [J]. Current Biotechnology, 2022, 12(2): 296-304. |
| [11] | LIU Shibo1, WU Hao1, HONG Jiao2, LIU Mengyu1, YAO Mawulikplimi Adzavon1, ZHAO Pengxiang1*. Advances in the Study of Inflammation-tumor Transformation in Ocular Diseases [J]. Curr. Biotech., 2020, 10(3): 234-241. |
| [12] | ZHANG Haitao, ZHANG Yuxin*. miR-148a Inhibits Glycolysis and Cell Proliferation of Breast Cancer Cells Through Targeted Regulation of Hexokinase 2 Gene [J]. Curr. Biotech., 2020, 10(2): 176-184. |
| [13] | HAN Chao1,2, ZOU Wei2, LIU Jing1*. T-type Calcium Channel and Breast Cancer [J]. journal1, 2012, 2(1): 29-33. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||