Current Biotechnology ›› 2025, Vol. 15 ›› Issue (4): 627-635.DOI: 10.19586/j.2095-2341.2025.0007
• Reviews • Previous Articles Next Articles
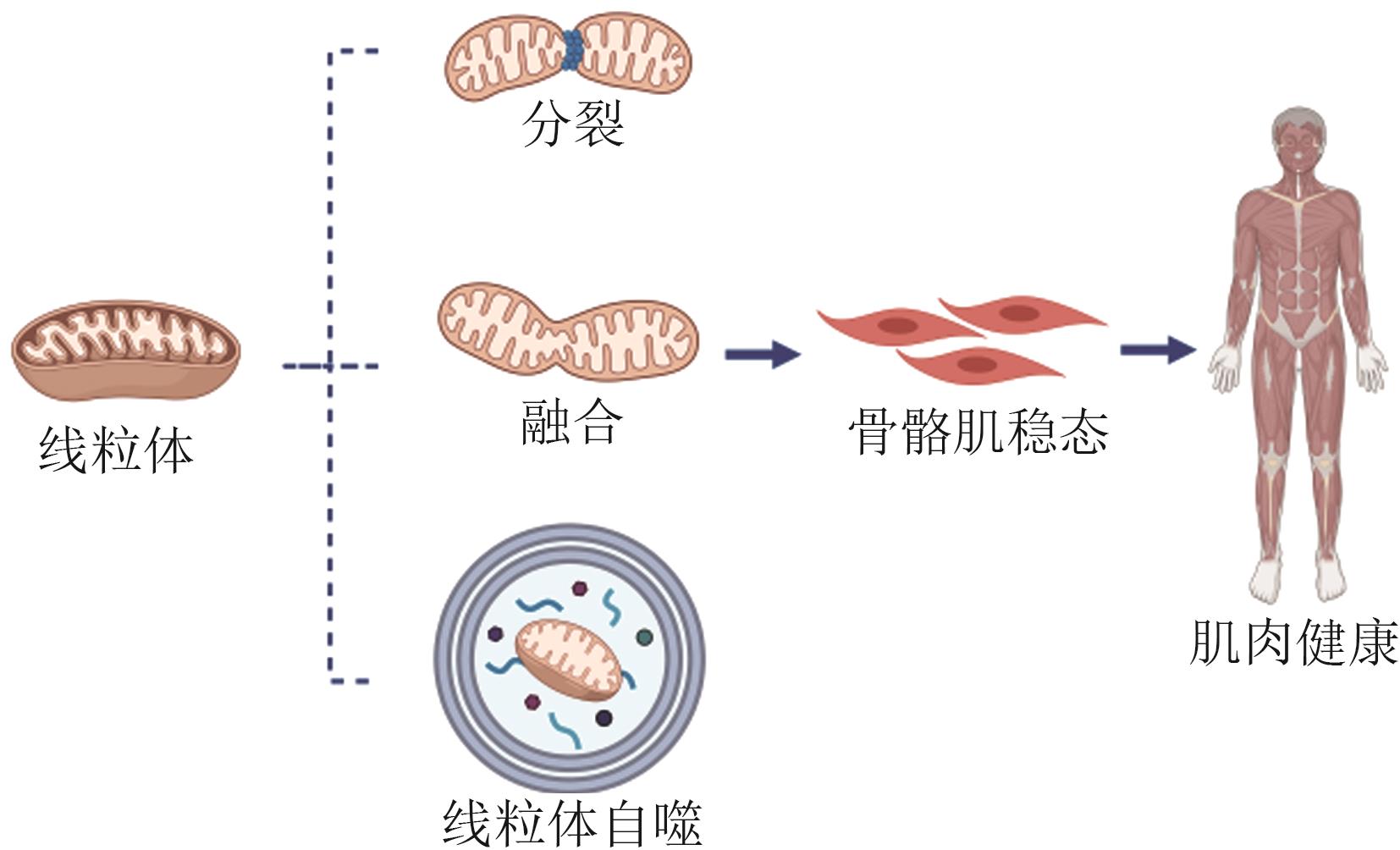
Research Progress on Mitochondria Maintaining Skeletal Muscle Homeostasis
Peixian GAO( ), Xueqi HAN, Jinyi ZHAO, Pengxiang ZHAO, Baihui ZHENG, Mengyu LIU(
), Xueqi HAN, Jinyi ZHAO, Pengxiang ZHAO, Baihui ZHENG, Mengyu LIU( )
)
- College of Chemistry and Life Sciences,Beijing University of Technology,Beijing 100124,China
-
Received:2025-01-20Accepted:2025-03-10Online:2025-07-25Published:2025-09-08 -
Contact:Mengyu LIU
线粒体维持骨骼肌稳态研究进展
高佩娴( ), 韩雪奇, 赵瑾宜, 赵鹏翔, 郑百卉, 刘梦昱(
), 韩雪奇, 赵瑾宜, 赵鹏翔, 郑百卉, 刘梦昱( )
)
- 北京工业大学化学与生命科学学院,北京 100124
-
通讯作者:刘梦昱 -
作者简介:高佩娴 E-mail: gaopeixian0104@163.com; -
基金资助:军委后勤保障部开放研究重点项目(BHJ17L08)
CLC Number:
Cite this article
Peixian GAO, Xueqi HAN, Jinyi ZHAO, Pengxiang ZHAO, Baihui ZHENG, Mengyu LIU. Research Progress on Mitochondria Maintaining Skeletal Muscle Homeostasis[J]. Current Biotechnology, 2025, 15(4): 627-635.
高佩娴, 韩雪奇, 赵瑾宜, 赵鹏翔, 郑百卉, 刘梦昱. 线粒体维持骨骼肌稳态研究进展[J]. 生物技术进展, 2025, 15(4): 627-635.
share this article
| [1] | SARTORI R, ROMANELLO V, SANDRI M. Mechanisms of muscle atrophy and hypertrophy: implications in health and disease[J/OL]. Nat. Commun., 2021, 12(1): 330[2025-02-27]. . |
| [2] | LEDUC-GAUDET J P, HUSSAIN S N A, BARREIRO E, et al.. Mitochondrial dynamics and mitophagy in skeletal muscle health and aging[J/OL]. Int. J. Mol. Sci., 2021, 22(15): 8179[2025-02-27]. . |
| [3] | YAN Y, LI M, LIN J, et al.. Adenosine monophosphate activated protein kinase contributes to skeletal muscle health through the control of mitochondrial function[J/OL]. Front. Pharmacol., 2022, 13: 947387[2025-02-27]. . |
| [4] | JAVADOV S, KOZLOV A V, CAMARA A K S. Mitochondria in health and diseases[J/OL]. Cells, 2020, 9(5): 1177[2025-02-27]. . |
| [5] | SAKELLARIOU G K, PEARSON T, LIGHTFOOT A P, et al.. Mitochondrial ROS regulate oxidative damage and mitophagy but not age-related muscle fiber atrophy[J/OL]. Sci. Rep., 2016, 6: 33944[2025-02-27]. . |
| [6] | ROMANELLO V, SANDRI M. The connection between the dynamic remodeling of the mitochondrial network and the regulation of muscle mass[J]. Cell. Mol. Life Sci., 2021, 78(4): 1305-1328. |
| [7] | MATSUMOTO C, SEKINE H, NAHATA M, et al.. Role of mitochondrial dysfunction in the pathogenesis of cisplatin-induced myotube atrophy[J]. Biol. Pharm. Bull., 2022, 45(6): 780-792. |
| [8] | MEMME J M, OLIVEIRA A N, HOOD D A. P53 regulates skeletal muscle mitophagy and mitochondrial quality control following denervation-induced muscle disuse[J/OL]. J. Biol. Chem., 2022, 298(2): 101540[2025-02-27]. . |
| [9] | ANDREUX P A, VAN DIEMEN M P J, HEEZEN M R, et al.. Publisher correction: mitochondrial function is impaired in the skeletal muscle of pre-frail elderly[J/OL]. Sci. Rep., 2019, 9(1): 17821[2025-02-27]. . |
| [10] | ISSEMANN I, GREEN S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators[J]. Nature, 1990, 347(6294): 645-650. |
| [11] | KONG S, CAI B, NIE Q. PGC-1α affects skeletal muscle and adipose tissue development by regulating mitochondrial biogenesis[J]. Mol. Genet. Genom., 2022, 297(3): 621-633. |
| [12] | QIAN L, ZHU Y, DENG C, et al.. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) family in physiological and pathophysiological process and diseases[J/OL]. Signal Transduct Target Ther., 2024, 9(1): 50[2025-02-27]. . |
| [13] | LIU C, LIN J D. PGC-1 coactivators in the control of energy metabolism[J]. Acta Biochim. Biophys. Sin., 2011, 43(4): 248-257. |
| [14] | CHEN S D, LIN T K, LIN J W, et al.. Activation of calcium/calmodulin-dependent protein kinase Ⅳ and peroxisome proliferator-activated receptor γ coactivator-1α signaling pathway protects against neuronal injury and promotes mitochondrial biogenesis in the hippocampal CA1 subfield after transient global ischemia[J]. J. Neurosci. Res., 2010, 88(14): 3144-3154. |
| [15] | ISLAM H, EDGETT B A, GURD B J. Coordination of mitochondrial biogenesis by PGC-1α in human skeletal muscle: a re-evaluation[J]. Metabolism, 2018, 79: 42-51. |
| [16] | FEALY C E, GREVENDONK L, HOEKS J, et al.. Skeletal muscle mitochondrial network dynamics in metabolic disorders and aging[J]. Trends Mol. Med., 2021, 27(11): 1033-1044. |
| [17] | PERNAS L, SCORRANO L. Mito-morphosis: mitochondrial fusion, fission, and cristae remodeling as key mediators of cellular function[J]. Annu. Rev. Physiol., 2016, 78: 505-531. |
| [18] | TWIG G, ELORZA A, MOLINA A J, et al.. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy[J]. Embo J., 2008, 27(2): 433-446. |
| [19] | OLICHON A, ELACHOURI G, BARICAULT L, et al.. OPA1 alternate splicing uncouples an evolutionary conserved function in mitochondrial fusion from a vertebrate restricted function in apoptosis[J]. Cell Death Differ., 2007, 14(4): 682-692. |
| [20] | ZHANG D, ZHANG Y, MA J, et al.. Cryo-EM structures of S-OPA1 reveal its interactions with membrane and changes upon nucleotide binding[J/OL]. eLife, 2020, 9: e50294[2025-02-27]. . |
| [21] | VON DER MALSBURG A, SAPP G M, ZUCCARO K E, et al.. Structural mechanism of mitochondrial membrane remodelling by human OPA1[J]. Nature, 2023, 620(7976): 1101-1108. |
| [22] | PALMER C S, OSELLAME L D, LAINE D, et al.. MiD49 and MiD51, new components of the mitochondrial fission machinery[J]. Embo Rep., 2011, 12(6): 565-573. |
| [23] | ZHAO J, LIU T, JIN S, et al.. Human MIEF1 recruits Drp1 to mitochondrial outer membranes and promotes mitochondrial fusion rather than fission[J]. Embo J., 2011, 30(14): 2762-2778. |
| [24] | LOSÓN O C, SONG Z, CHEN H, et al.. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission[J]. Mol. Biol. Cell, 2013, 24(5): 659-667. |
| [25] | CHATZINIKITA E, MARIDAKI M, PALIKARAS K, et al.. The role of mitophagy in skeletal muscle damage and regeneration[J/OL]. Cells, 2023, 12(5): 716[2025-02-27]. . |
| [26] | RILEY B E, LOUGHEED J C, CALLAWAY K, et al.. Structure and function of Parkin E3 ubiquitin ligase reveals aspects of RING and HECT ligases[J/OL]. Nat. Commun., 2013, 4: 1982[2025-02-27]. . |
| [27] | GEISLER S, HOLMSTRÖM K M, SKUJAT D, et al.. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1[J]. Nat. Cell Biol., 2010, 12(2): 119-131. |
| [28] | LAZAROU M, SLITER D A, KANE L A, et al.. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy[J]. Nature, 2015, 524(7565): 309-314. |
| [29] | WU W, LIN C, WU K, et al.. FUNDC1 regulates mitochondrial dynamics at the ER-mitochondrial contact site under hypoxic conditions[J]. Embo J., 2016, 35(13): 1368-1384. |
| [30] | CHEN H, DETMER S A, EWALD A J, et al.. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development[J]. J. Cell Biol., 2003, 160(2): 189-200. |
| [31] | BACH D, PICH S, SORIANO F X, et al.. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism a novel regulatory mechanism altered in obesity[J]. J. Biol. Chem., 2003, 278(19): 17190-17197. |
| [32] | HOUZELLE A, JÖRGENSEN J A, SCHAART G, et al.. Human skeletal muscle mitochondrial dynamics in relation to oxidative capacity and insulin sensitivity[J]. Diabetologia, 2021, 64(2): 424-436. |
| [33] | ZHENG P, MA W, GU Y, et al.. High-fat diet causes mitochondrial damage and downregulation of mitofusin-2 and optic atrophy-1 in multiple organs[J]. J. Clin. Biochem. Nutr., 2023, 73(1): 61-76. |
| [34] | SPIEGEL R, SAADA A, FLANNERY P J, et al.. Fatal infantile mitochondrial encephalomyopathy, hypertrophic cardiomyopathy and optic atrophy associated with a homozygous OPA1 mutation[J]. J. Med. Genet., 2016, 53(2): 127-131. |
| [35] | DULAC M, PLEDUC G J, REYNAUD O, et al.. Drp1 knockdown induces severe muscle atrophy and remodelling, mitochondrial dysfunction, autophagy impairment and denervation[J]. J. Physiol., 2020, 598(17): 3691-3710. |
| [36] | YASUDA T, ISHIHARA T, ICHIMURA A, et al.. Mitochondrial dynamics define muscle fiber type by modulating cellular metabolic pathways[J/OL]. Cell Rep., 2023, 42(5): 112434[2025-02-27]. . |
| [37] | FAVARO G, ROMANELLO V, VARANITA T, et al.. DRP1-mediated mitochondrial shape controls calcium homeostasis and muscle mass[J/OL]. Nat. Commun., 2019, 10(1): 2576[2025-02-27]. . |
| [38] | LEE T T, CHEN P L, SU M P, et al.. Loss of Fis1 impairs proteostasis during skeletal muscle aging in Drosophila [J/OL]. Aging Cell, 2021, 20(6): e13379[2025-02-27]. . |
| [39] | ZHANG Z, SLITER D A, BLECK C K E, et al.. Fis1 deficiencies differentially affect mitochondrial quality in skeletal muscle[J]. Mitochondrion, 2019, 49: 217-226. |
| [40] | PEKER N, DONIPADI V, SHARMA M, et al.. Loss of Parkin impairs mitochondrial function and leads to muscle atrophy[J]. Am. J. Physiol. Cell Physiol., 2018, 315(2): 164-185. |
| [41] | GOUSPILLOU G, GODIN R, PIQUEREAU J, et al.. Protective role of Parkin in skeletal muscle contractile and mitochondrial function[J]. J. Physiol., 2018, 596(13): 2565-2579. |
| [42] | ITO A, HASHIMOTO M, TANIHATA J, et al.. Involvement of Parkin-mediated mitophagy in the pathogenesis of chronic obstructive pulmonary disease-related sarcopenia[J]. J. Cachexia Sarcopenia Muscle, 2022, 13(3): 1864-1882. |
| [43] | LEDUC-GAUDET J P, MAYAKID, REYNAUDO, et al.. Parkin overexpression attenuates sepsis-induced muscle wasting[J/OL]. Cells, 2020, 9(6): 1454[2025-02-27. . |
| [44] | AXELROD C L, FEALY C E, MULYA A, et al.. Exercise training remodels human skeletal muscle mitochondrial fission and fusion machinery towards a pro-elongation phenotype[J/OL]. Acta Physiol., 2019, 225(4): e13216[2025-02-27]. . |
| [45] | FU T, XU Z, LIU L, et al.. Mitophagy directs muscle-adipose crosstalk to alleviate dietary obesity[J]. Cell Rep., 2018, 23(5): 1357-1372. |
| [46] | SUNTAR I, SUREDA A, BELWAL T, et al.. Natural products, PGC-1α, and Duchenne muscular dystrophy[J]. Acta Pharm. Sin. B, 2020, 10(5): 734-745. |
| [47] | LIN J, WU H, TARR P T, et al.. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres[J]. Nature, 2002, 418(6899): 797-801. |
| [48] | ROWE G C, PATTEN I S, ZSENGELLER Z K, et al.. Disconnecting mitochondrial content from respiratory chain capacity in PGC-1-deficient skeletal muscle[J]. Cell Rep., 2013, 3(5): 1449-1456. |
| [49] | ROBERTS-WILSON T K, REDDY R N, BAILEY J L, et al.. Calcineurin signaling and PGC-1 alpha expression are suppressed during muscle atrophy due to diabetes[J]. Biochim. Biophys. Acta, 2010, 1803(8): 960-967. |
| [50] | SATO K, SATOSHI Y, MIYAUCHI Y, et al.. Downregulation of PGC-1α during cisplatin-induced muscle atrophy in murine skeletal muscle[J/OL]. Biochim. Biophys. Acta Mol. Basis Dis., 2024, 1870(1): 166877[2025-02-27]. . |
| [51] | GARCIA S, NISSANKA N, MARECO E A, et al.. Overexpression of PGC-1α in aging muscle enhances a subset of young-like molecular patterns[J/OL]. Aging Cell, 2018, 17(2): e12707[2025-02-27]. . |
| [52] | KIM H, CHO S C, JEONG H J, et al.. Indoprofen prevents muscle wasting in aged mice through activation of PDK1/AKT pathway[J]. J. Cachexia Sarcopenia Muscle, 2020, 11(4): 1070-1088. |
| [53] | BALAN E, SCHWALM C, NASLAIN D, et al.. Regular endurance exercise promotes fission, mitophagy, and oxidative phosphorylation in human skeletal muscle independently of age[J/OL]. Physiol, 2019, 10: 1088[2025-02-27]. . |
| [54] | MUSCI R V, ANDRIE K M, WALSH M A, et al.. Phytochemical compound PB125 attenuates skeletal muscle mitochondrial dysfunction and impaired proteostasis in a model of musculoskeletal decline[J]. J. Physiol., 2023, 601(11): 2189-2216. |
| [55] | HUANG D, YUE F, QIU J, et al.. Polymeric nanoparticles functionalized with muscle-homing peptides for targeted delivery of phosphatase and tensin homolog inhibitor to skeletal muscle[J]. Acta Biomater., 2020, 118: 196-206. |
| [56] | THEILEN N T, JEREMIC N, WEBER G J, et al.. TFAM overexpression diminishes skeletal muscle atrophy after hindlimb suspension in mice[J]. Arch. Biochem. Biophys., 2019, 666: 138-147. |
| [1] | Bingying QIU, Xueyao CHEN, Hui WANG, Chenhong LI, Dongsheng ZHANG. Rapid Acquisition of Avian Mitochondrial Genome Based on Gene Capture Method [J]. Current Biotechnology, 2024, 14(4): 618-630. |
| [2] | Yuqi WANG, Xinyu WANG, Yingfan WANG, Yuancui MENG, Xizhang YAN, Pan CHANG. Research Progress of Mitochondrial Biosynthesis in Diabetes Cardiomyopathy [J]. Current Biotechnology, 2024, 14(2): 221-227. |
| [3] | Lanlan ZHANG, Caihua LI, Yuzhu FANG, Yan SONG, Wanlin KANG, Zhiyu LI, Xiao ZHANG, Rui ZHANG. Progress on the Application of Mitochondrial SSR Molecular Markers in Plants [J]. Current Biotechnology, 2023, 13(6): 821-826. |
| [4] | Zhou PAN, Ke HU. The Role of Mitochondrial Dysfunction in Hypoxic Pulmonary Hypertension [J]. Current Biotechnology, 2023, 13(6): 882-888. |
| [5] | Weiping ZHANG, Bingying QIU, Dongsheng ZHANG. Adaptive Evolution Analysis of Mitochondrial Genomes in Anseriform Birds with Various Feeding Habits [J]. Current Biotechnology, 2023, 13(5): 748-754. |
| [6] | Chen MA, Yifei SONG, Yang YI, Ziyi LIU, Fei XIE, Xuemei MA. Research Progress on the Relationship Between Hydrogen and Mitochondria [J]. Current Biotechnology, 2023, 13(3): 366-374. |
| [7] | Xiao ZHANG, Caihua LI, Jing WANG, Lanlan ZHANG, Xiaoyu MOU, Xinyue WANG, Liumei GAN, Pengzhan ZHOU, Rui ZHANG. Impact of Gene Duplication on RNA Editing Rates of Mitochondrial atpA Gene in Cotton (Gossypium hirsutum L.) [J]. Current Biotechnology, 2022, 12(5): 737-745. |
| [8] | Kaiyao HOU, Erfei ZHANG, Lina ZHENG, Hongguang CHEN, Keliang XIE. The Effect of Hydrogen-rich Saline on Mitochondrial Autophagy of Myocardial Cells in Sepsis-induced Mice [J]. Current Biotechnology, 2022, 12(4): 497-502. |
| [9] | MA Xuemei1, ZHANG Xin1, XIE Fei1, ZHAO Pengxiang1, ZHANG Zhao1, YI Yang1, ZHANG Xiaokang1, MA Shengnan1, LI Qinjian1, LYU Baobei1, LIU Mengyu, YAO Mawulikplimi Adzavon1, SUN Xuejun2, LI Yingxian3. Bio-enzyme Basis of Hydrogen in Biological System [J]. Curr. Biotech., 2020, 10(1): 15-22. |
| [10] | WANG Peilin, ZHOU Lili, LIANG Chengzhen, MENG Zhigang, GUO Sandui*, ZHANG Rui*. Improvement of Cotton Mitochondrial Gene cRT-PCR and its Application in Searching for CMS Related Genes [J]. Curr. Biotech., 2019, 9(3): 303-308. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||