Current Biotechnology ›› 2024, Vol. 14 ›› Issue (3): 422-432.DOI: 10.19586/j.2095-2341.2024.0013
• Articles • Previous Articles Next Articles
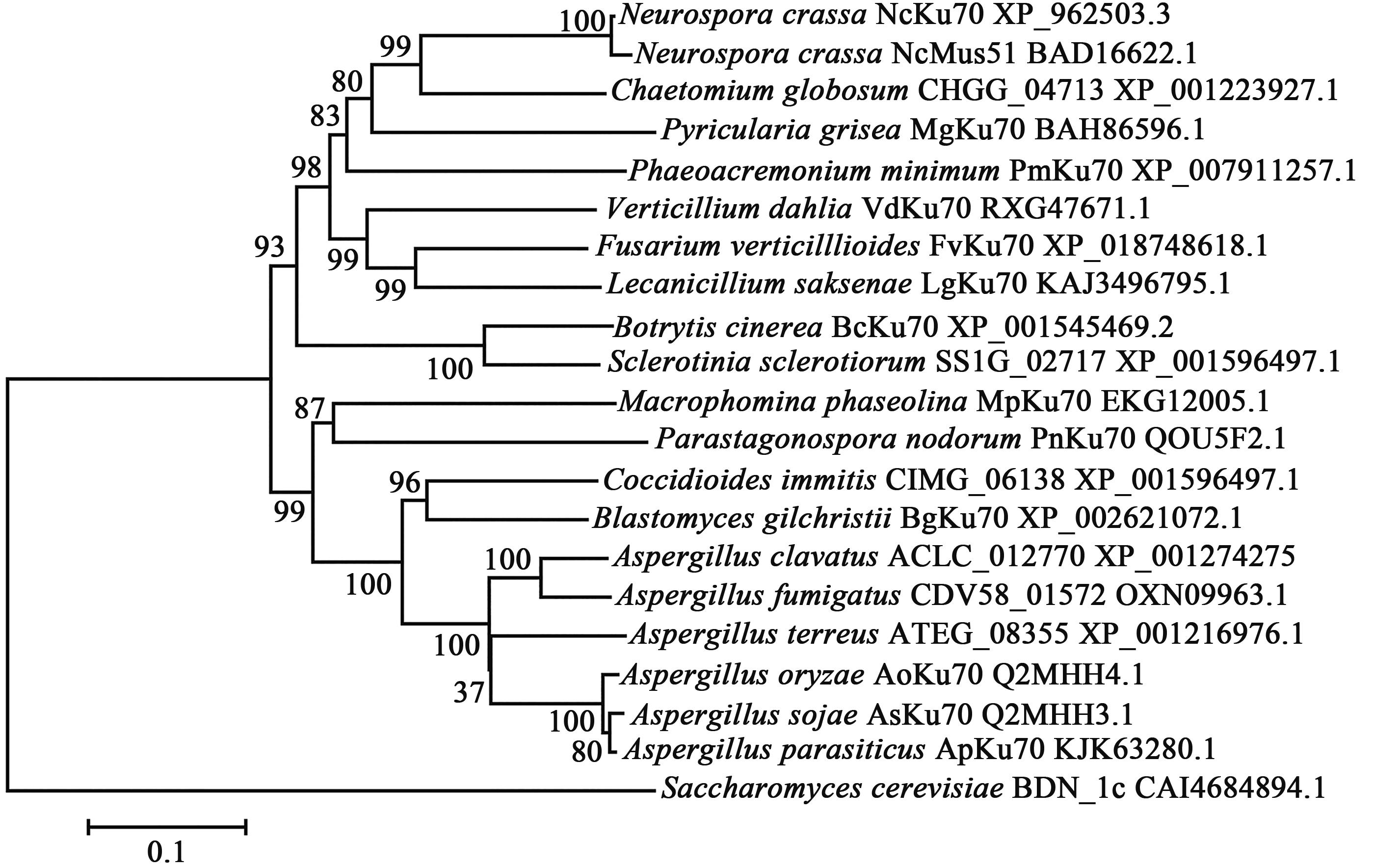
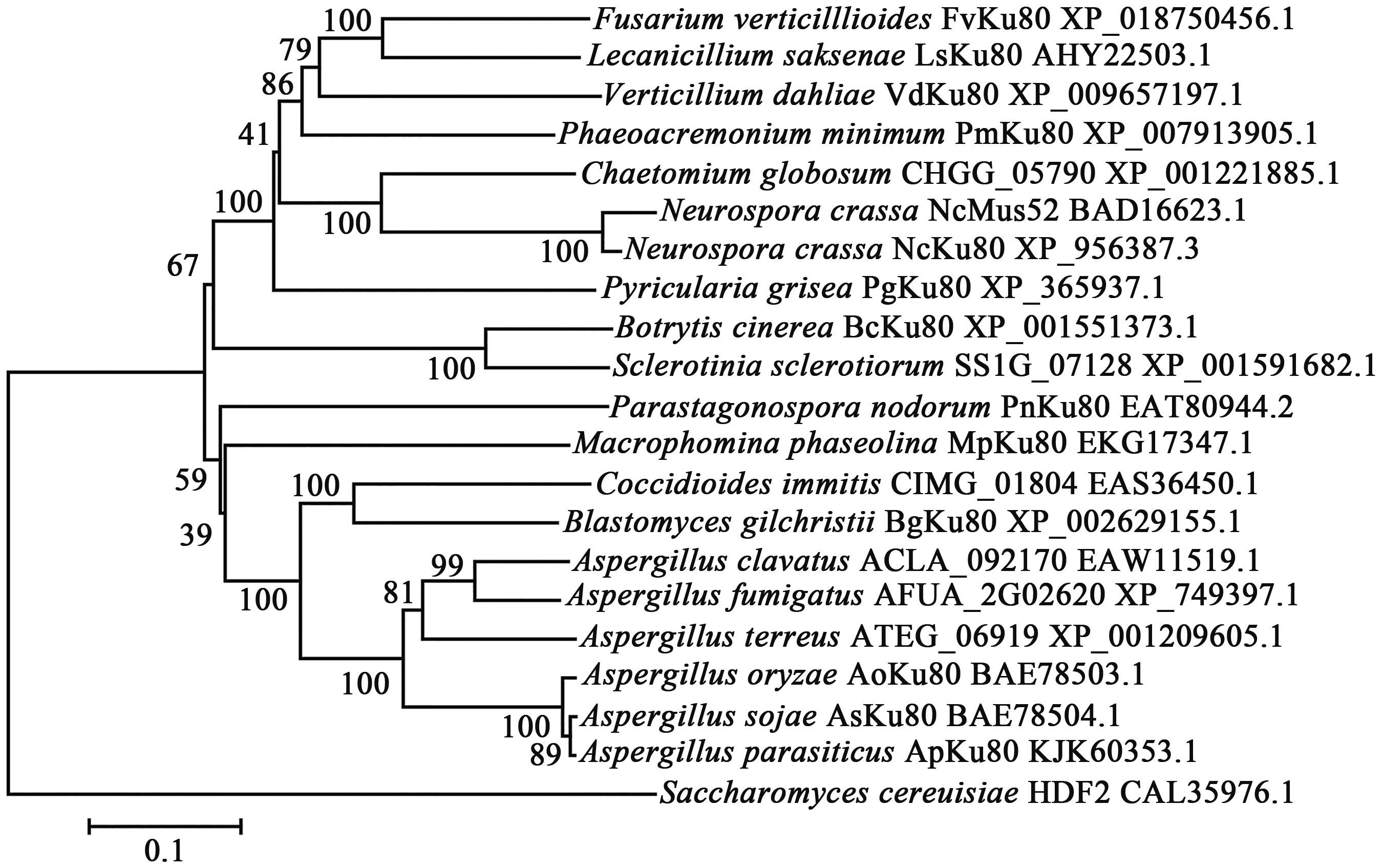
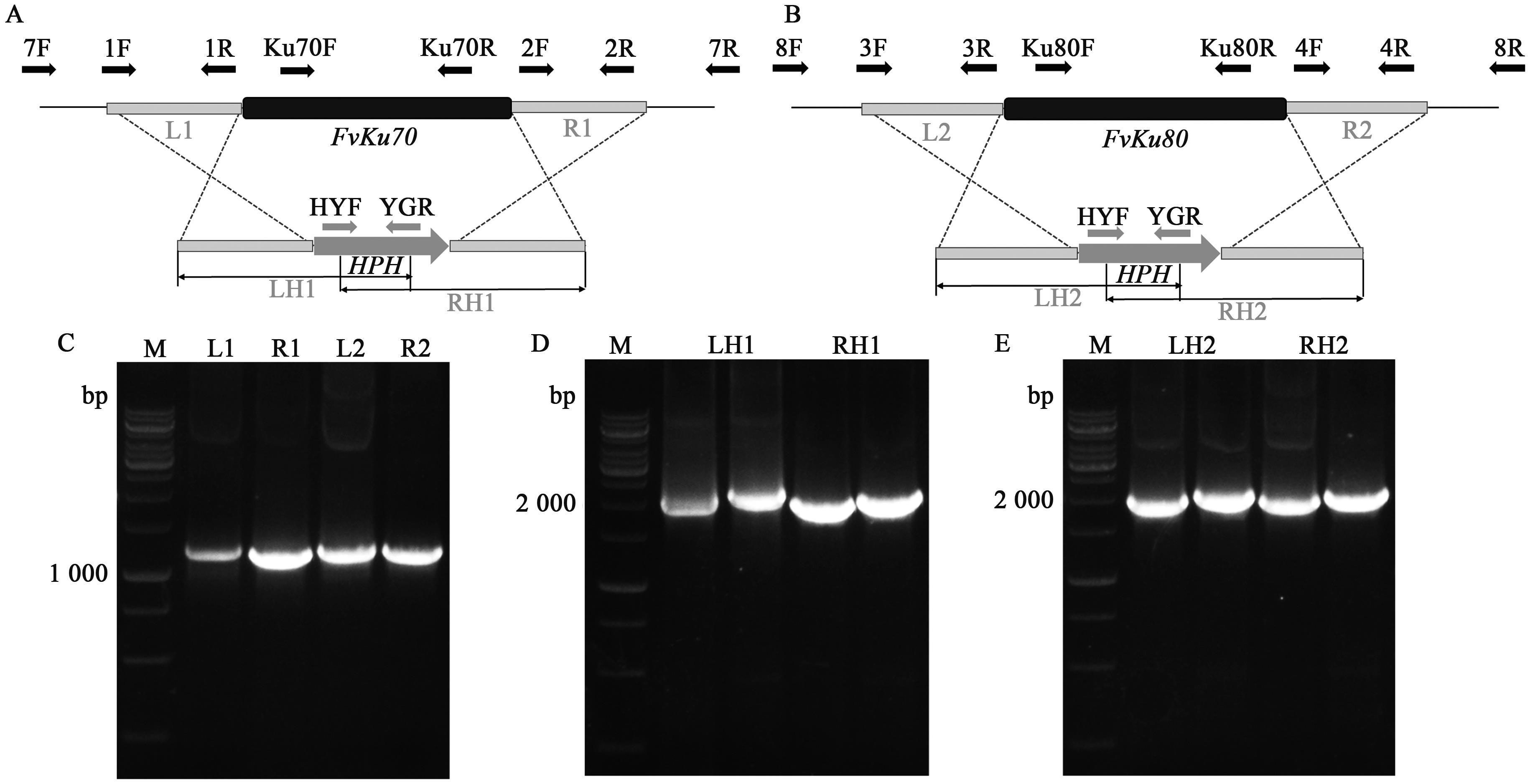
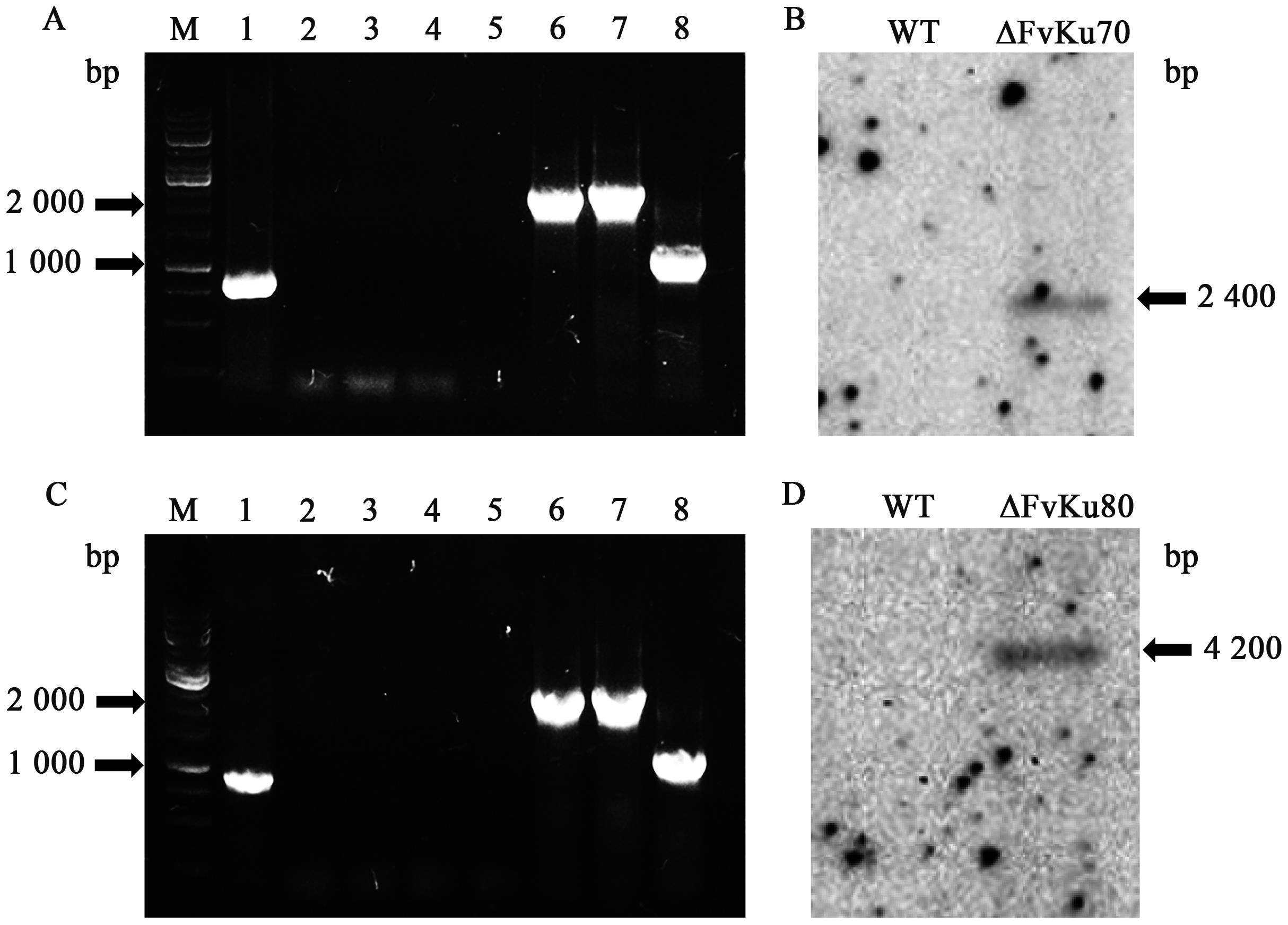
Highly Efficient Gene Knockout Method in Fusarium verticillioides Using Nonhomologous End-joining Deficiency
Kaifei XI( ), Chengjie LI, Yi DING, Wei GUO(
), Chengjie LI, Yi DING, Wei GUO( )
)
- Institute of Food Science and Technology,Chinese Academy of Agricultural Sciences,Beijing 100193,China
-
Received:2024-01-23Accepted:2024-02-27Online:2024-05-25Published:2024-06-18 -
Contact:Wei GUO
利用非同源末端连接缺陷构建拟轮枝镰孢菌的高效基因敲除方法
- 中国农业科学院农产品加工研究所,北京 100193
-
通讯作者:郭维 -
作者简介:席凯飞 E-mail: kaifeix@163.com; -
基金资助:国家自然科学基金项目(32072377);中国农业科学院科技创新工程所重点任务(CAAS-ASTIP-G2022-IFST-01)
CLC Number:
Cite this article
Kaifei XI, Chengjie LI, Yi DING, Wei GUO. Highly Efficient Gene Knockout Method in Fusarium verticillioides Using Nonhomologous End-joining Deficiency[J]. Current Biotechnology, 2024, 14(3): 422-432.
席凯飞, 李成杰, 丁艺, 郭维. 利用非同源末端连接缺陷构建拟轮枝镰孢菌的高效基因敲除方法[J]. 生物技术进展, 2024, 14(3): 422-432.
share this article
| 引物 | 引物序列(5’→3’) |
|---|---|
| 1F | 5’-GATTACGAATTCGAGCTCGGTACCACTCCTTGTTGGTCTTGCCT-3’ |
| 1R | 5’-ATCTCTAGAGGATCCCCGGGTACCATTGATACGATTGATGTCTG-3’ |
| 2F | 5’-GTCGACCTGCAGGCATGCAAGCTTGACTGAGGTCGAAGGGCAAA-3’ |
| 2R | 5’-AAAACGACGGCCAGTGCCAAGCTTAATATGTAAAAGTCCAACCC-3’ |
| 3F | 5’-GATTACGAATTCGAGCTCGGTACCCTCCGATACCAAGCCAGCA-3’ |
| 3R | 5’-ATCTCTAGAGGATCCCCGGGTACCTCATACCCATCATCGTCTTG-3’ |
| 4F | 5’-GTCGACCTGCAGGCATGCAAGCTTGAGTGAATCATAGGGCCTTG-3’ |
| 4R | 5’-AAAACGACGGCCAGTGCCAAGCTTACATCTCTGGGTGTTGCGAA-3’ |
| 7F | 5’-GGGTAAGGATCACCTTGATA-3’ |
| 7R | 5’-TTAAAGGCAATCGCGATCGA-3’ |
| 8F | 5’-TCCATCAGCATACCACTCCT-3’ |
| 8R | 5’-ATAGACTCGAAGATGGTGAG-3’ |
| HYR | 5’-GTATTGACCGATTCCTTGCGGTCCGAA-3’ |
| YGF | 5’-GATGTAGGAGGGCGTGGATATGTCCT-3’ |
| HYF | 5’-ATGAAAAAGCCTGAACTC-3’ |
| YGR | 5’-TTCCTTTGCCCTCGGACG-3’ |
| Hyg-F | 5’-ATGAAAAAGCCTGAACTCAC-3’ |
| Hyg-R | 5’-CTATTCCTTTGCCCTCGGAC-3’ |
| Ku70F | 5’-GATGACTTGGGTGACATTTC-3’ |
| Ku70R | 5’-GTCCAAAGGAGGAAACTGGT-3’ |
| Ku80F | 5’-GGAGACGAGAAATTCGACCT-3’ |
| Ku80R | 5’-CTGAAGAATTCTGTAGTGCC-3’ |
| pyrG-up-F | 5’-GATTACGAATTCGAGCTCGGTACCCATAAAAACCATGAACCATT-3’ |
| pyrG-up-R | 5’-ATCTCTAGAGGATCCCCGGGTACCGAGGTGAGAGGTGAGAGGTG-3’ |
| pyrG-dw-F | 5’-GTCGACCTGCAGGCATGCAAGCTTTGGAAGTCGGGACGGCCTTG-3’ |
| pyrG-dw-R | 5’-AAAACGACGGCCAGTGCCAAGCTTAGCCTTGTACCTTTCCTTGA-3’ |
| pyrG-u1300-F | 5’-TAACCGTCTCAGACCTGAAAG-3’ |
| pyrG-d1300-R | 5’-TCGGATAGTACTGCCAGTTGA-3’ |
| pyrG-in-F | 5’-CAAGGCCTCGGTTGCATCTC-3’ |
| pyrG-in-R | 5’-CCATGTCATAATGCGTCTTGA-3’ |
| pyrG-F/ | 5’-CGACATTCCACCCATTTACGC-3’ |
| pyrG-R | 5’-GACGTGGTGAATCGGCCGACT-3’ |
Table 1 Primers used in this study
| 引物 | 引物序列(5’→3’) |
|---|---|
| 1F | 5’-GATTACGAATTCGAGCTCGGTACCACTCCTTGTTGGTCTTGCCT-3’ |
| 1R | 5’-ATCTCTAGAGGATCCCCGGGTACCATTGATACGATTGATGTCTG-3’ |
| 2F | 5’-GTCGACCTGCAGGCATGCAAGCTTGACTGAGGTCGAAGGGCAAA-3’ |
| 2R | 5’-AAAACGACGGCCAGTGCCAAGCTTAATATGTAAAAGTCCAACCC-3’ |
| 3F | 5’-GATTACGAATTCGAGCTCGGTACCCTCCGATACCAAGCCAGCA-3’ |
| 3R | 5’-ATCTCTAGAGGATCCCCGGGTACCTCATACCCATCATCGTCTTG-3’ |
| 4F | 5’-GTCGACCTGCAGGCATGCAAGCTTGAGTGAATCATAGGGCCTTG-3’ |
| 4R | 5’-AAAACGACGGCCAGTGCCAAGCTTACATCTCTGGGTGTTGCGAA-3’ |
| 7F | 5’-GGGTAAGGATCACCTTGATA-3’ |
| 7R | 5’-TTAAAGGCAATCGCGATCGA-3’ |
| 8F | 5’-TCCATCAGCATACCACTCCT-3’ |
| 8R | 5’-ATAGACTCGAAGATGGTGAG-3’ |
| HYR | 5’-GTATTGACCGATTCCTTGCGGTCCGAA-3’ |
| YGF | 5’-GATGTAGGAGGGCGTGGATATGTCCT-3’ |
| HYF | 5’-ATGAAAAAGCCTGAACTC-3’ |
| YGR | 5’-TTCCTTTGCCCTCGGACG-3’ |
| Hyg-F | 5’-ATGAAAAAGCCTGAACTCAC-3’ |
| Hyg-R | 5’-CTATTCCTTTGCCCTCGGAC-3’ |
| Ku70F | 5’-GATGACTTGGGTGACATTTC-3’ |
| Ku70R | 5’-GTCCAAAGGAGGAAACTGGT-3’ |
| Ku80F | 5’-GGAGACGAGAAATTCGACCT-3’ |
| Ku80R | 5’-CTGAAGAATTCTGTAGTGCC-3’ |
| pyrG-up-F | 5’-GATTACGAATTCGAGCTCGGTACCCATAAAAACCATGAACCATT-3’ |
| pyrG-up-R | 5’-ATCTCTAGAGGATCCCCGGGTACCGAGGTGAGAGGTGAGAGGTG-3’ |
| pyrG-dw-F | 5’-GTCGACCTGCAGGCATGCAAGCTTTGGAAGTCGGGACGGCCTTG-3’ |
| pyrG-dw-R | 5’-AAAACGACGGCCAGTGCCAAGCTTAGCCTTGTACCTTTCCTTGA-3’ |
| pyrG-u1300-F | 5’-TAACCGTCTCAGACCTGAAAG-3’ |
| pyrG-d1300-R | 5’-TCGGATAGTACTGCCAGTTGA-3’ |
| pyrG-in-F | 5’-CAAGGCCTCGGTTGCATCTC-3’ |
| pyrG-in-R | 5’-CCATGTCATAATGCGTCTTGA-3’ |
| pyrG-F/ | 5’-CGACATTCCACCCATTTACGC-3’ |
| pyrG-R | 5’-GACGTGGTGAATCGGCCGACT-3’ |
| 1 | KAMLE M, MAHATO D K, DEVI S, et al.. Fumonisins: impact on agriculture, food, and human health and their management strategies[J/OL]. Toxins, 2019, 11(6): 328[2023-10-20]. . |
| 2 | JONES R E, HUMPHREY T C. Homologous recombination and nonhomologous end-joining repair in yeast[M]. 2nd ed. New York: Academic Press, 2019: 2715-2726. |
| 3 | HABER J E. Transpositions and translocations induced by site-specific double-strand breaks in budding yeast[J]. DNA Repair, 2006, 5(9-10): 998-1009. |
| 4 | KITO H, FUJIKAWA T, MORIWAKI A, et al.. MgLig4, a homolog of Neurospora crassa Mus-53 (DNA ligase IV), is involved in, but not essential for, non-homologous end-joining events in Magnaporthe grisea [J]. Fungal Genet. Biol., 2008, 45(12): 1543-1551. |
| 5 | XU D, ZHAO H. Pathway choice for DNA double strand break repair[J]. Sci. Sin. Vitae, 2021, 51(1): 56-69. |
| 6 | GUHA S, BHAUMIK S R. Transcription-coupled DNA double-strand break repair[J/OL]. DNA Repair, 2022, 109: 103211[2024-04-08]. . |
| 7 | D'ADDA DI FAGAGNA F, HANDE M P, TONG W M, et al.. Effects of DNA nonhomologous end-joining factors on telomere length and chromosomal stability in mammalian cells[J]. Curr. Biol., 2001, 11(15): 1192-1196. |
| 8 | PRYOR J M, CONLIN M P, CARVAJAL-GARCIA J, et al.. Ribonucleotide incorporation enables repair of chromosome breaks by nonhomologous end joining[J]. Science, 2018, 361(6407): 1126-1129. |
| 9 | NINOMIYA Y, SUZUKI K, ISHII C, et al.. Highly efficient gene replacements in Neurospora strains deficient for nonhomologous end-joining[J]. Proc. Natl. Acad. Sci. USA, 2004, 101(33): 12248-12253. |
| 10 | WEYDA I, YANG L, VANG J, et al.. A comparison of Agrobacterium-mediated transformation and protoplast-mediated transformation with CRISPR/Cas9 and bipartite gene targeting substrates, as effective gene targeting tools for Aspergillus carbonarius [J]. J. Microbiol. Meth., 2017, 135: 26-34. |
| 11 | KRAPPMANN S, SASSE C, BRAUS G H. Gene targeting in Aspergillus fumigatus by homologous recombination is facilitated in a nonhomologous end-joining-deficient genetic background[J]. Eukaryot. Cell, 2006, 5(1): 212-215. |
| 12 | MEYER V, ARENTSHORST M, EL-GHEZAL A, et al.. Highly efficient gene targeting in the Aspergillus niger kusA mutant[J]. J. Biotechnol., 2007, 128(4): 770-775. |
| 13 | VILLALBA F, COLLEMARE J, LANDRAUD P, et al.. Improved gene targeting in Magnaporthe grisea by inactivation of MgKU80 required for non-homologous end joining[J]. Fungal Genet. Biol., 2008, 45(1): 68-75. |
| 14 | CHANG P K, SCHARFENSTEIN L L, WEI Q, et al.. Development and refinement of a high-efficiency gene-targeting system for Aspergillus flavus [J]. J. Microbiol. Meth., 2010, 81(3): 240-246. |
| 15 | PÖGGELER S, KÜCK U. Highly efficient generation of signal transduction knockout mutants using a fungal strain deficient in the mammalian Ku70 ortholog[J]. Gene, 2006, 378: 1-10. |
| 16 | LI Z H, DU C M, ZHONG Y H, et al.. Development of a highly efficient gene targeting system allowing rapid genetic manipulations in Penicillium decumbens [J]. Appl. Microbiol. Biotechnol., 2010, 87(3): 1065-1076. |
| 17 | XI K, SHAN L, YANG Y, et al.. Species diversity and chemotypes of Fusarium species associated with maize stalk rot in Yunnan Province of Southwest China[J/OL]. Front. Microbiol., 2021, 12: 652062[2021-10-20]. . |
| 18 | MARTINS M P, GOMES E V, SANCHES P R, et al.. Mus-52 disruption and metabolic regulation in Neurospora crassa: transcriptional responses to extracellular phosphate availability[J/OL]. PLoS ONE, 2018, 13(4): e0195871[2023-10-30]. . |
| 19 | ZHOU J, LI S M. Conversion of viridicatic acid to crustosic acid by cytochrome P450 enzyme-catalysed hydroxylation and spontaneous cyclisation[J]. Appl. Microbiol. Biotechnol., 2021, 105(24): 9181-9189. |
| 20 | FENG J, LI W, HWANG S F, et al.. Enhanced gene replacement frequency in KU70 disruption strain of Stagonospora nodorum [J]. Microbiol. Res., 2012, 167(3): 173-178. |
| 21 | HOFF B, KAMEREWERD J, SIGL C, et al.. Homologous recombination in the antibiotic producer Penicillium chrysogenum: strain DeltaPcku70 shows up-regulation of genes from the HOG pathway[J]. Appl. Microbiol. Biotechnol., 2010, 85(4): 1081-1094. |
| 22 | TAKAHASHI T, MASUDA T, KOYAMA Y. Enhanced gene targeting frequency in Ku70 and Ku80 disruption mutants of Aspergillus sojae and Aspergillus oryzae [J]. Mol. Genet. Genom., 2006, 275(5): 460-470. |
| 23 | 许铭,尹志远,高明煜,等.苹果树腐烂病菌高效基因敲除受体菌株ΔVmKu80的构建[J].西北农业学报,2016,25(2):298-305. |
| XU M, YIN Z Y, GAO M Y, et al.. Construction of enhanced gene deletion frequency recipient strain ΔVmKu80 in Valsa mali[J]. Acta Agric. Boreali Occidentalis Sin., 2016, 25(2): 298-305. | |
| 24 | QI X, SU X, GUO H, et al.. A Ku70 null mutant improves gene targeting frequency in the fungal pathogen Verticillium dahliae [J]. World J. Microbiol. Biotechnol., 2015, 31(12): 1889-1897. |
| 25 | HAARMANN T, LORENZ N, TUDZYNSKI P. Use of a nonhomologous end joining deficient strain (Deltaku70) of the ergot fungus Claviceps purpurea for identification of a nonribosomal peptide synthetase gene involved in ergotamine biosynthesis[J]. Fungal Genet. Biol., 2008, 45(1): 35-44. |
| 26 | WANG H, PERRAULT A R, TAKEDA Y, et al.. Biochemical evidence for Ku-independent backup pathways of NHEJ[J/OL]. Nucleic Acids Res., 2020, 48(9): 5200[2023-10-20]. . |
| 27 | DAI Z, POMRANING K R, DENG S, et al.. Deletion of the KU70 homologue facilitates gene targeting in Lipomyces starkeyi strain NRRL Y-11558[J]. Curr. Genet., 2019, 65(1): 269-282. |
| [1] | Licun LIANG, Wenlong LIU, Xiaoqing LIU, Bin YAO, Huoqing HUANG, Haomeng YANG. The Effect of Chemical Reagents on the Efficiency of Gene Editing in Aspergillus tubingensis [J]. Current Biotechnology, 2024, 14(4): 586-593. |
| [2] | LI Wei-wei1,2, ZHANG Ji1, CHEN Chao-ru1, WANG Zhi1, QU Juan-juan2*, DUN Bao-qing1*, LI Gui-ying1, LU Ming1. Research on the Effects of CIT2 Gene on Xylose Utilization of the Recombinant Saccharomyces cerevisiae [J]. Curr. Biotech., 2016, 6(6): 455-461. |
| [3] | GAO Li, CHEN Yan, HU Ting-mao, LI Guang-peng*. The Latest Research Progress of Myostatin in Mammals [J]. Curr. Biotech., 2014, 4(6): 381-388. |
| [4] | SU Yue-yan, WANG Xu-jing, WANG Zhi-xing*. Research Progress on Marker-free Plant Transgenic Technology [J]. Curr. Biotech., 2013, 3(3): 157-161. |
| [5] | XIAO Shui-hua1,2, HONG Qin-yang1,3, LIN Yan-zhen1,2, LI Jin-yu1,2*. Research and Application of Gene Knockout Technology in Bacillus [J]. Curr. Biotech., 2013, 3(2): 81-84. |
| [6] | ZHANG Chen, ZHOU Zheng-fu, CHEN Ming, LIN Min, ZHANG Wei*. Research Progress on DNA Repair Mode and Functional Proteins Involved in the Extreme Radiation Resistant Deinococcus radiodurans [J]. Curr. Biotech., 2013, 3(2): 103-108. |
| [7] | ZHANG Ya-xu. Research Summary on DNA Recombination Technology [J]. journal1, 2012, 2(1): 57-63. |
| [8] | ZHANG Xiao-juan, WEN Yin*. Ku Gene in Microorganisms [J]. journal1, 2011, 1(1): 26-31. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||