Current Biotechnology ›› 2024, Vol. 14 ›› Issue (1): 72-84.DOI: 10.19586/j.2095-2341.2023.0111
• Special Forum on Development and Technology of Biologics • Previous Articles Next Articles
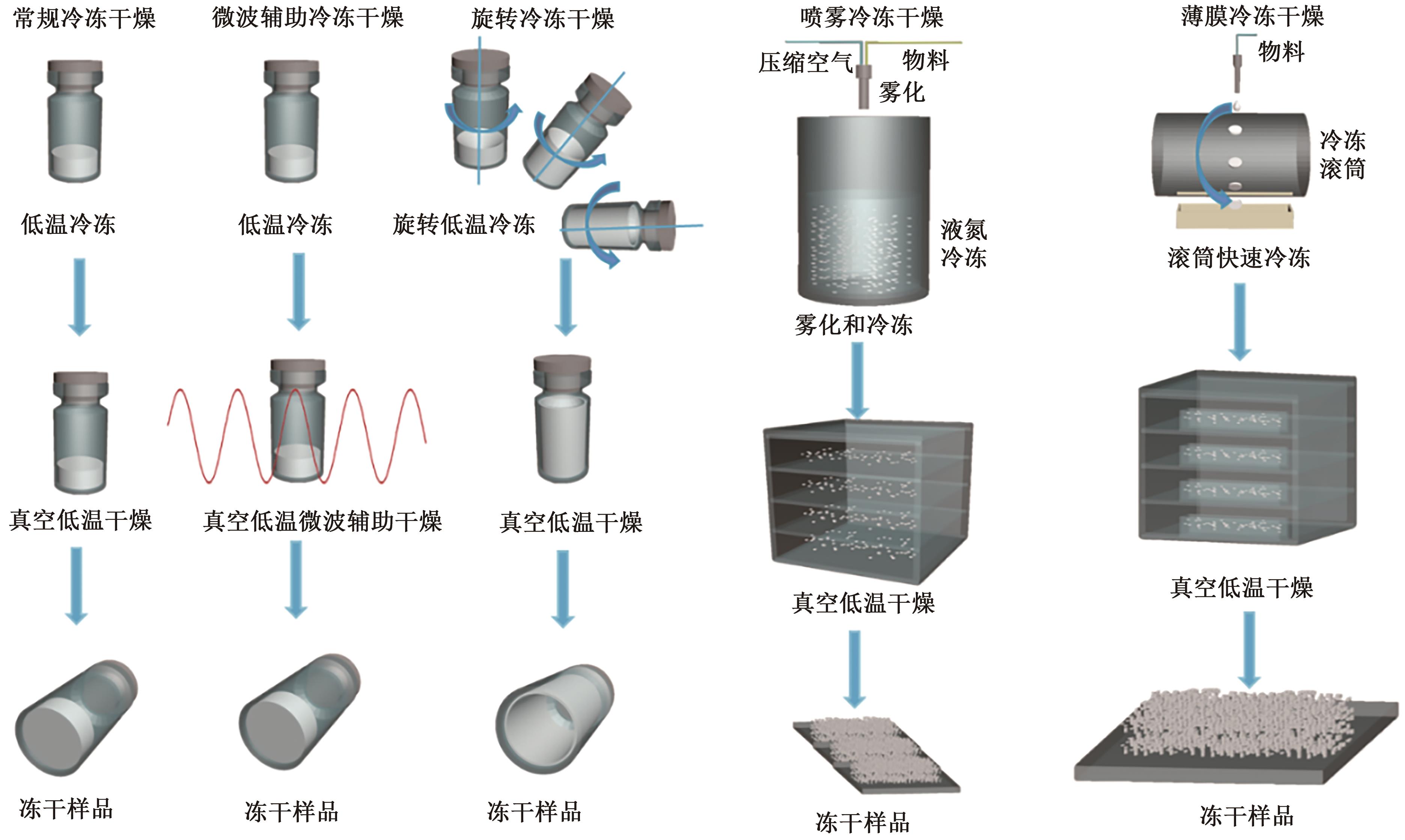
The Application Progress of Freeze-drying Technology in Vaccine Research and Devolopment
Ronglin LIU1( ), Ning WANG2, Yanyi LI1(
), Ning WANG2, Yanyi LI1( ), Weiting ZHANG1, Chu LUO1, Weibing NIU1
), Weiting ZHANG1, Chu LUO1, Weibing NIU1
- 1.North China Pharmaceutical Jintan Biotechnology Co. ,Ltd. ,Shijiazhuang 050035,China
2.Shijiazhuang Agricultural Technology Extension Center,Shijiazhuang 050000,China
-
Received:2023-09-18Accepted:2023-11-15Online:2024-01-25Published:2024-02-05 -
Contact:Yanyi LI
冷冻干燥技术在疫苗研发中的应用进展
刘容麟1( ), 王宁2, 李岩异1(
), 王宁2, 李岩异1( ), 张卫婷1, 罗楚1, 牛维兵1
), 张卫婷1, 罗楚1, 牛维兵1
- 1.华北制药金坦生物技术股份有限公司,石家庄 050035
2.石家庄市农业技术推广中心,石家庄 050000
-
通讯作者:李岩异 -
作者简介:刘容麟 E-mail: iuronglin6@163.com;
CLC Number:
Cite this article
Ronglin LIU, Ning WANG, Yanyi LI, Weiting ZHANG, Chu LUO, Weibing NIU. The Application Progress of Freeze-drying Technology in Vaccine Research and Devolopment[J]. Current Biotechnology, 2024, 14(1): 72-84.
刘容麟, 王宁, 李岩异, 张卫婷, 罗楚, 牛维兵. 冷冻干燥技术在疫苗研发中的应用进展[J]. 生物技术进展, 2024, 14(1): 72-84.
share this article
| 疫苗 | 疫苗类型 | 冻干方式 | 稳定性 | 使用配方 | 参考文献 |
|---|---|---|---|---|---|
| 重组牛痘疫苗(rTTV-OVA) | 病毒载体疫苗 | 冷冻干燥 | 在4 ℃和25 ℃均能提高热稳定性,保持了良好的免疫反应性和免疫原性 | 聚乙二醇∶右旋糖酐∶牛血清白蛋白= 50∶5∶4 | [ |
| 人5型腺病毒疫苗(Ad5-ENV) | 病毒载体疫苗 | 冷冻干燥 | 在4 ℃和25 ℃均能提高热稳定性,保持了良好的感染性和免疫原性 | 聚乙二醇∶L-谷氨酰胺=50∶9 | [ |
| 口服轮状疫苗-默克公司RotaTeq® | 减毒活疫苗 | 冷冻干燥 | 5 ℃下稳定超过36个月,在37 ℃下稳定20个月,在45 ℃下稳定7个月仍然保持良好疫苗效力 | HSRV04D5配方 | [ |
| 新型冠状病毒疫苗 | 核酸疫苗 | 冷冻干燥 | 冻干后脂质体封装完整,在4、22和37 ℃条件至少可以保持12周且转染性无明显变化 | 脂质体∶mRNA=20∶1;12.5%蔗糖、Tris或磷酸盐 | [ |
| 四价流感疫苗Fluad® | 基因重组疫苗 | 薄膜冷冻干燥 | 重组血凝素(rHA)抗原的完整性和血凝活性,冻干制剂对重复冻融不敏感,具有良好的稳定性 | 蔗糖 | [ |
| 牛合并病毒灭活疫苗(pneumo-5) | 灭活疫苗 | 冷冻干燥 | 5种病毒:牛病毒性腹泻病毒(BVDV)1和2型、牛单纯疱疹病毒1.1型(BoHV-1.1)、牛流感病毒3型(BPSV)和牛呼吸道合胞病毒(BRSV)均能够保护小牛,并且具有安全性和有效性 | — | [ |
| 炭疽疫苗(anthrax vaccine) | 减毒活疫苗 | 冷冻干燥 | 20 ℃储存条件下可以在180 d内保持高效力 | — | [ |
| 风疹疫苗(rubella vaccine) | 减毒活疫苗 | 冷冻干燥 | 与商用的明胶稳定剂配方对比,表现出充分的稳定性 | 海藻糖 | [ |
| 腮腺炎活疫苗(mumps vaccine RS-12 株) | 减毒活疫苗 | 冷冻干燥 | 在37 ℃条件下存储1周,CCID50下降幅度不到10倍 | 海藻糖二水合物 | [ |
| 带状疱疹病毒疫苗 | 减毒活疫苗 | 冷冻干燥 | 临床接种无论年龄、性别或合并症,都能有效降低被接种者患上带状疱疹的风险 | — | [ |
| 黄热病毒(vYF) | 减毒活疫苗 | 喷雾干燥 | 具有良好的断裂力和高抗溃散性;2~8 ℃下冷藏3年,这种干燥微粒的vYF感染滴度与冷冻干燥产品相似,都具有良好的稳定性;在25 ℃和37 ℃的加速稳定性研究中,微粒中vYF的降解动力学与常规冻干产品无显著差异 | 糖/聚合物 | [ |
| 结核分枝杆菌疫苗 | 亚单位疫苗 | 冷冻干燥 | 45 ℃条件下加热1周后进行免疫,舌下抗体反应并未减弱 | 糖类和佐剂 | [ |
| 水痘带状疱疹病毒疫苗(VZV) | 亚单位疫苗 | 冷冻干燥 | 体液免疫和细胞免疫反应方面均表现出极高的效果,稳定性良好 | CIA09A的新型脂质体佐剂 | [ |
| 埃博拉病毒疫苗(EBOV) | 重组病毒载体疫苗 | 冷冻干燥 | 40 ℃下稳定12周,且仍保持免疫原性 | 9.5%海藻糖为稳定剂,乙酸铵调节冻干过程中的离子强度 | [ |
| 新型冠状病毒疫苗 | 核酸疫苗 | 冷冻干燥 | 疫苗在25 ℃条件下保持6个月的物理化学性质和生物活性稳定,可以产生强大的免疫效应,且没有严重不良事件 | 脂质、mRNA | [ |
| 新型冠状病毒疫苗 | 核酸疫苗 | 冷冻干燥 | -20 ℃储存条件下至少能保持12个月,无转染效果损失 | 乳酸-共-缩-乙二醇、mRNA | [ |
| 人乳头瘤病毒(HPV MS2-L2) | 病毒样颗粒疫苗 | 喷雾干燥 | 37 ℃条件下可以存放2个月仍保持良好的免疫效果,所产生的抗体持久性也很好,在10个月期间没有明显的衰减 | - | [ |
| 二价诺如病毒疫苗(norovirus) | 病毒样颗粒疫苗 | 薄膜冷冻干燥 | 冻干粉在40 ℃、相对湿度为75%条件下储存8周后,抗原的效力仍保持在指定的可接受范围内 | 4.55%或5.55%蔗糖,或3%~4%海藻糖加上0.55%蔗糖 | [ |
| 肺炎球菌疫苗(pneumococcal vaccine) | 结合类疫苗 | 冷冻干燥 | 冻干过程中疫苗的凝聚和效力无降低,热稳定性良好 | 0.5%丙二醇和6%甘露醇、0.5%羧甲基纤维素钠和4%蔗糖 | [ |
Table 1 Examples of freeze-dried vaccines and their stability
| 疫苗 | 疫苗类型 | 冻干方式 | 稳定性 | 使用配方 | 参考文献 |
|---|---|---|---|---|---|
| 重组牛痘疫苗(rTTV-OVA) | 病毒载体疫苗 | 冷冻干燥 | 在4 ℃和25 ℃均能提高热稳定性,保持了良好的免疫反应性和免疫原性 | 聚乙二醇∶右旋糖酐∶牛血清白蛋白= 50∶5∶4 | [ |
| 人5型腺病毒疫苗(Ad5-ENV) | 病毒载体疫苗 | 冷冻干燥 | 在4 ℃和25 ℃均能提高热稳定性,保持了良好的感染性和免疫原性 | 聚乙二醇∶L-谷氨酰胺=50∶9 | [ |
| 口服轮状疫苗-默克公司RotaTeq® | 减毒活疫苗 | 冷冻干燥 | 5 ℃下稳定超过36个月,在37 ℃下稳定20个月,在45 ℃下稳定7个月仍然保持良好疫苗效力 | HSRV04D5配方 | [ |
| 新型冠状病毒疫苗 | 核酸疫苗 | 冷冻干燥 | 冻干后脂质体封装完整,在4、22和37 ℃条件至少可以保持12周且转染性无明显变化 | 脂质体∶mRNA=20∶1;12.5%蔗糖、Tris或磷酸盐 | [ |
| 四价流感疫苗Fluad® | 基因重组疫苗 | 薄膜冷冻干燥 | 重组血凝素(rHA)抗原的完整性和血凝活性,冻干制剂对重复冻融不敏感,具有良好的稳定性 | 蔗糖 | [ |
| 牛合并病毒灭活疫苗(pneumo-5) | 灭活疫苗 | 冷冻干燥 | 5种病毒:牛病毒性腹泻病毒(BVDV)1和2型、牛单纯疱疹病毒1.1型(BoHV-1.1)、牛流感病毒3型(BPSV)和牛呼吸道合胞病毒(BRSV)均能够保护小牛,并且具有安全性和有效性 | — | [ |
| 炭疽疫苗(anthrax vaccine) | 减毒活疫苗 | 冷冻干燥 | 20 ℃储存条件下可以在180 d内保持高效力 | — | [ |
| 风疹疫苗(rubella vaccine) | 减毒活疫苗 | 冷冻干燥 | 与商用的明胶稳定剂配方对比,表现出充分的稳定性 | 海藻糖 | [ |
| 腮腺炎活疫苗(mumps vaccine RS-12 株) | 减毒活疫苗 | 冷冻干燥 | 在37 ℃条件下存储1周,CCID50下降幅度不到10倍 | 海藻糖二水合物 | [ |
| 带状疱疹病毒疫苗 | 减毒活疫苗 | 冷冻干燥 | 临床接种无论年龄、性别或合并症,都能有效降低被接种者患上带状疱疹的风险 | — | [ |
| 黄热病毒(vYF) | 减毒活疫苗 | 喷雾干燥 | 具有良好的断裂力和高抗溃散性;2~8 ℃下冷藏3年,这种干燥微粒的vYF感染滴度与冷冻干燥产品相似,都具有良好的稳定性;在25 ℃和37 ℃的加速稳定性研究中,微粒中vYF的降解动力学与常规冻干产品无显著差异 | 糖/聚合物 | [ |
| 结核分枝杆菌疫苗 | 亚单位疫苗 | 冷冻干燥 | 45 ℃条件下加热1周后进行免疫,舌下抗体反应并未减弱 | 糖类和佐剂 | [ |
| 水痘带状疱疹病毒疫苗(VZV) | 亚单位疫苗 | 冷冻干燥 | 体液免疫和细胞免疫反应方面均表现出极高的效果,稳定性良好 | CIA09A的新型脂质体佐剂 | [ |
| 埃博拉病毒疫苗(EBOV) | 重组病毒载体疫苗 | 冷冻干燥 | 40 ℃下稳定12周,且仍保持免疫原性 | 9.5%海藻糖为稳定剂,乙酸铵调节冻干过程中的离子强度 | [ |
| 新型冠状病毒疫苗 | 核酸疫苗 | 冷冻干燥 | 疫苗在25 ℃条件下保持6个月的物理化学性质和生物活性稳定,可以产生强大的免疫效应,且没有严重不良事件 | 脂质、mRNA | [ |
| 新型冠状病毒疫苗 | 核酸疫苗 | 冷冻干燥 | -20 ℃储存条件下至少能保持12个月,无转染效果损失 | 乳酸-共-缩-乙二醇、mRNA | [ |
| 人乳头瘤病毒(HPV MS2-L2) | 病毒样颗粒疫苗 | 喷雾干燥 | 37 ℃条件下可以存放2个月仍保持良好的免疫效果,所产生的抗体持久性也很好,在10个月期间没有明显的衰减 | - | [ |
| 二价诺如病毒疫苗(norovirus) | 病毒样颗粒疫苗 | 薄膜冷冻干燥 | 冻干粉在40 ℃、相对湿度为75%条件下储存8周后,抗原的效力仍保持在指定的可接受范围内 | 4.55%或5.55%蔗糖,或3%~4%海藻糖加上0.55%蔗糖 | [ |
| 肺炎球菌疫苗(pneumococcal vaccine) | 结合类疫苗 | 冷冻干燥 | 冻干过程中疫苗的凝聚和效力无降低,热稳定性良好 | 0.5%丙二醇和6%甘露醇、0.5%羧甲基纤维素钠和4%蔗糖 | [ |
| 1 | CHEN Y, LIAO Q, CHEN T, et al.. Freeze-drying formulations increased the adenovirus and poxvirus vaccine storage times and antigen stabilities[J]. Virol. Sin., 2021, 36(3): 365-372. |
| 2 | LYU J L, BAO L R, SHEN X, et al.. The preparation of N-IgY targeting SARS-CoV-2 and its immunomodulation to IFN-gamma production in vitro[J/OL]. Int. Immunopharmacol., 2021, 96: 107797[2021-06-25]. . |
| 3 | HARRIS R J. Preservation of biological materials by freeze-drying[J]. Nature, 1951, 168(4281): 851-853. |
| 4 | WANG Z, LI L, REN G, et al.. A comprehensive review on stability of therapeutic proteins treated by freeze-drying: induced stresses and stabilization mechanisms involved in processing[J]. Dry. Technol., 2022, 40(16): 3373-3388. |
| 5 | LIU Y, ZHANG Z, HU L. High efficient freeze-drying technology in food industry[J]. Crit. Rev. Food Sci. Nutr., 2022, 62(12): 3370-3388. |
| 6 | FRANKS F. Freeze-drying of bioproducts: putting principles into practice[J]. Eur. J. Pharm. Biopharm., 1998, 45(3): 221-229. |
| 7 | WANG G Q, PU J, YU X Q, et al.. Influence of freezing temperature before freeze-drying on the viability of various Lactobacillus plantarum strains[J]. J. Dairy Sci., 2020, 103(4): 3066-3075. |
| 8 | SITAR A, ŠKRLEC K, VOGLAR J, et al.. Effects of controlled nucleation on freeze-drying lactose and mannitol aqueous solutions[J]. Dry. Technol., 2018, 36(10): 1263-1272. |
| 9 | NUYTTEN G, REVATTA S R, VAN BOCKSTAL P, et al.. Development and application of a mechanistic cooling and freezing model of the spin freezing step within the sramework of continuous freeze-drying[J/OL]. Rmaceutics, 2021, 13: 2076[2021-12-29]. . |
| 10 | KAWASAKI H, SHIMANOUCHI T, KIMURA Y. Recent development of optimization of lyophilization process[J/OL]. J. Chem., 2019, 2019: 9502856[2019-05-05]. . |
| 11 | WANG Z, DUAN X, LI L, et al.. Effects of freeze-drying and microwave vacuum freeze-drying on the activity of IgY: from the perspective of protein structure[J]. Dry. Technol., 2023, 41(2): 222-232. |
| 12 | ASSEGEHEGN G, FUENTE B D L, FRANCO J, et al.. Understanding and optimization of the secondary drying step of a freeze-drying process: a case study[J]. Dry. Technol., 2021, 39(8): 1003-1017. |
| 13 | YOON K, NARSIMHAN V. Understanding heat transfer during the secondary drying stage of freeze drying: current practice and knowledge gaps[J]. J. Pharm. Sci., 2022, 111(2): 368-381. |
| 14 | MEULEWAETER S, NUYTTEN G, CHENG M H Y, et al.. Continuous freeze-drying of messenger RNA lipid nanoparticles enables storage at higher temperatures[J/OL]. J. Control. Release, 2023, 357: 149[2023-03-21]. . |
| 15 | HUANG Y, ZHENG S, GUO Z, et al.. Ionizable liposomal siRNA therapeutics enables potent and persistent treatment of Hepatitis B[J/OL]. Signal Transduct. Target. Ther., 2022, 7: 38[2022-02-12]. . |
| 16 | MATEJTSCHUK P, BIRD C, EZEAJUGHI E, et al.. Impact of formulation choices on the freeze-drying of an interleukin-6 reference material[J/OL]. Front. Mol. Biosci., 2022, 9: 868460[2023-12-20]. . |
| 17 | MADAN M, SIKRIWAL D, SHARMA G, et al.. Rational design of heat stable lyophilized rotavirus vaccine formulations[J]. Hum. Vaccines Immunother., 2018, 14(9): 2132-2141. |
| 18 | MEYER L D, VAN BOCKSTAL P J, CORVER J, et al.. Evaluation of spin freezing versus conventional freezing as part of a continuous pharmaceutical freeze-drying concept for unit doses[J]. Int. J. Pharm., 2015, 496(1): 75-85. |
| 19 | LEYS L, NUYTTEN G, VAN BOCKSTAL P J, et al.. Evaluation of a PAT-based in-line control system for a continuous spin freeze-drying process[J/OL]. Int. J. Pharm., 2023, 641: 123062[2023-05-21]. . |
| 20 | Method and system for freeze-drying injectable compositions, in particular pharmaceutival compositions : US201815922757[P] 2019-03-01. |
| 21 | LUY B, STAMATO H. Spray freeze drying[M]//OHTAKE S, IZUTSU K, LECHUGA-BALLESTEROS D. Drying technologies for biotechnology and pharmaceutical applications. America: John Wiley & Sons Ltd, 2020, 217-237. |
| 22 | DI A, ZHANG S, LIU X, et al.. Microfluidic spray dried and spray freeze dried uniform microparticles potentially for intranasal drug delivery and controlled release[J]. Powder Technol., 2021, 379: 144-153. |
| 23 | SHOKOUH M K, FAGHIHI H, DARABI M, et al.. Formulation and evaluation of inhalable microparticles of Rizatriptan benzoate processed by spray freeze-drying[J/OL]. J. Drug Deliv. Sci. Tec., 2021, 62: 7[2019-05-05]. . |
| 24 | QIU Y, MAN R C H, LIAO Q, et al.. Effective mRNA pulmonary delivery by dry powder formulation of PEGylated synthetic KL4 peptide[J]. J. Control. Release Off. J. Control. Release Soc., 2019, 314: 102-115. |
| 25 | MUTUKURI T T, DARWISH A, STRONGRICH A D, et al.. Radio frequency-assisted ultrasonic spray freeze drying for pharmaceutical protein solids[J]. J. Pharm. Sci., 2023, 112(1): 40-50. |
| 26 | PAN H W, SEOW H C, LO J C K, et al.. Spray-dried and spray-freeze-dried powder formulations of an anti-interleukin-4Rα antibody for pulmonary delivery[J]. Pharm. Res., 2022, 39(9): 2291-2304. |
| 27 | YU S H, PU X H, AHMED M U, et al.. Spray-freeze-dried inhalable composite microparticles containing nanoparticles of combinational drugs for potential treatment of lung infections caused by Pseudomonas aeruginosa [J/OL]. Int. J. Pharmaceut., 2021, 610: 9[2021-10-01]. . |
| 28 | DORETH M, HUSSEIN M A, PRIEMEL P A, et al.. Amorphization within the tablet: using microwave irradiation to form a glass solution in situ [J]. Int. J. Pharm., 2017, 519(1-2): 343-351. |
| 29 | GITTER J H, GEIDOBLER R, PRESSER I, et al.. Microwave-assisted freeze-drying of monoclonal antibodies: product quality aspects and storage stability[J/OL]. Pharmaceutics, 2019, 11(12):674[2019-12-18]. . |
| 30 | HARDTER N, GEIDOBLER R, PRESSER I, et al.. Accelerated production of biopharmaceuticals via microwave-assisted freeze-drying (MFD)[J/OL]. Pharmaceutics, 2023, 15: 15[2023-04-27]. . |
| 31 | YU Y S, ABOULFOTOUH K, XU H, et al.. Feasibility of intranasal delivery of thin-film freeze-dried, mucoadhesive vaccine powders[J/OL]. Int. J. Pharm., 2023, 640: 122990[2023-12-20]. . |
| 32 | HUFNAGEL S, XU H, SAHAKIJPIJARN S, et al.. Dry powders for inhalation containing monoclonal antibodies made by thin-film freeze-drying[J/OL]. Int. J. Pharm., 2022, 618: 121637[2022-03-09]. . |
| 33 | ABOUL FOTOUH K, UNO N, XU H, et al.. Formulation of dry powders of vaccines containing MF59 or AddaVax by thin-film freeze-drying: towards a dry powder universal flu vaccine[J/OL]. Int. J. Pharm., 2022, 624: 122021[2022-07-17]. . |
| 34 | YAN J K, WU L X, QIAO Z R, et al.. Effect of different drying methods on the product quality and bioactive polysaccharides of bitter gourd (Momordica charantia L.) slices[J]. Food Chem., 2019, 271: 588-596. |
| 35 | MEHANNA M M, ABLA K K. Recent advances in freeze-drying: variables, cycle optimization, and innovative techniques[J]. Pharm. Dev. Technol., 2022, 27(8): 904-923. |
| 36 | GUO Y, BALDELLI A, SINGH A, et al.. Production of high loading insulin nanoparticles suitable for oral delivery by spray drying and freeze drying techniques[J/OL]. Sci. Rep., 2022, 12: 9949[2022-06-15]. . |
| 37 | FADEEL M R A, EL-DAKHLY A T, ALLAM A M, et al.. Preparation and efficacy of freeze-dried inactivated vaccine against bovine viral diarrhea virus genotypes 1 and 2, bovine herpes virus type 1.1, bovine parainfluenza-3 virus, and bovine respiratory syncytial virus[J]. Clin. Exp. Vaccine Res., 2020, 9(2): 119-125. |
| 38 | 姚舜禹,张丽琳.新城疫疫苗研究进展[J].生物技术进展,2020,10(5):470-478. |
| YAO S Y, ZHANG L L. Progress on Newcastle disease vaccine[J]. Curr. Biotechnol., 2020, 10(5): 470-478. | |
| 39 | TYRRELL D A, RIDGWELL B. Freeze-drying of certain viruses[J]. Nature, 1965, 206: 115-116. |
| 40 | ABAYNEH T, GETACHEW B, GELAYE E, et al.. Viability evaluation of freeze dried and suspension anthrax spore vaccine formulations stored at different temperatures[J]. Vaccine, 2021, 39(42): 6245-6249. |
| 41 | SHOKRI S, SHAHKARAMI M K, SHAFYI A, et al.. Evaluation of the thermal stability of live-attenuated Rubella vaccine (Takahashi strain) formulated and lyophilized in different stabilizers[J]. J. Virol. Meth., 2019, 264: 18-22. |
| 42 | JAMIL R K, TAQAVIAN M, SADIGH Z A, et al.. Evaluation of the thermal stability of a novel strain of live-attenuated mumps vaccine (RS-12 strain) lyophilized in different stabilizers[J]. J. Virol. Meth., 2014, 199: 35-38. |
| 43 | MATSUMOTO K, OHFUJI S, INOHARA K, et al.. Effectiveness of live attenuated varicella-zoster vaccine in adults older than 50 years in Japan: a retrospective cohort study[J/OL]. Vaccines, 2023, 11: 9[2023-01-25]. . |
| 44 | CLÉNET D, HOURQUET V, WOINET B, et al.. A spray freeze dried micropellet based formulation proof-of-concept for a yellow fever vaccine candidate[J]. Eur. J. Pharm. Biopharm. 2019, 142: 334-343. |
| 45 | KELLY S H, OPOLOT E E, WU Y Y, et al.. Tabletized supramolecular assemblies for sublingual peptide immunization[J/OL]. Adv. Healthc. Mater., 2021, 10: 6[2021-02-01]. . |
| 46 | WUI S R, KIM K S, RYU J I, et al.. Efficient induction of cell-mediated immunity to varicella-zoster virus glycoprotein E co-lyophilized with a cationic liposome-based adjuvant in mice[J]. Vaccine, 2019, 37(15): 2131-2141. |
| 47 | ARNEMO M, VIKSMOEN WATLE S S, SCHOULTZ K M, et al.. Stability of a vesicular stomatitis virus-vectored Ebola vaccine[J]. J. Infect. Dis., 2016, 213(6): 930-933. |
| 48 | PRESTON K B, MONTICELLO C R, WONG T A S, et al.. Preservation of quaternary structure in thermostable, lyophilized filovirus glycoprotein vaccines: a search for stability-indicating assays[J]. J. Pharm. Sci., 2020, 109(12): 3716-3727. |
| 49 | PACKER M, GYAWALI D, YERABOLU R, et al.. A novel mechanism for the loss of mRNA activity in lipid nanoparticle delivery systems[J/OL]. Nat. Commun., 2021, 12: 6777 [2021-11-24]. . |
| 50 | AI L, LI Y, ZHOU L, et al.. Lyophilized mRNA-lipid nanoparticle vaccines with long-term stability and high antigenicity against SARS-CoV-2[J/OL]. Cell Discov., 2023, 9: 9[2023-01-23]. . |
| 51 | LI Z Y, ZHANG X Q, HO W, et al.. Lipid-polymer hybrid "particle-in-particle" nanostructure gene delivery platform explored for lyophilizable DNA and mRNA COVID-19 vaccines[J/OL]. Adv. Funct. Mater., 2022, 32: 16 [2022-07-22]. . |
| 52 | 李岩异,吕娜,贾思凝,等.体外组装的病毒样颗粒在疫苗和药物递送中的应用[J].生物技术进展,2023,13(2):201-209. |
| LI Y Y, LYU N, JIA S N, et al.. Application of virus like particles assembled in vitro in vaccine and drug delivery[J]. Curr. Biotechnol., 2023, 13(2): 201-209. | |
| 53 | YADAV R, ZHAI L, KUNDA N K, et al.. Mixed bacteriophage MS2-L2 VLPs elicit long-lasting protective antibodies against HPV pseudovirus 51[J/OL]. Viruses, 2021, 13(6):1113[2021-07-03]. . |
| 54 | XU H, BHOWMIK T, GONG K, et al.. Thin-film freeze-drying of a bivalent Norovirus vaccine while maintaining the potency of both antigens[J/OL]. Int. J. Pharm., 2021, 609: 121126[2023-12-20]. . |
| 55 | MENSCH C, CHINTALA R, NAWROCKI D, et al.. Enabling lyophilized pneumococcal conjugate vaccines through formulation design and excipient selection suitable for A multivalent adjuvanted vaccine[J]. J. Pharm. Sci., 2021, 110(1): 97-107. |
| [1] | Yanhua LIU, Rui GUO, Yanyi LI, Jinli CHEN. Progress on the Development of Vaccines Related to Chronic Respiratory Diseases [J]. Current Biotechnology, 2025, 15(3): 396-403. |
| [2] | Jian QIAO, Shuai ZENG, Yang ZHANG, Gelin XU. Application of Recombinant Trypsin in Tissue Isolation of Primary Hamster Kidney Cells [J]. Current Biotechnology, 2025, 15(1): 152-157. |
| [3] | Weili ZHAO, Na LYU, Huiqiang LI, Yahui HE, Lulu LI, Mingli LIANG, Yanyi LI. Research Progress of Virus-like Particles Vaccine [J]. Current Biotechnology, 2024, 14(5): 776-784. |
| [4] | Yaping HU, Zhenghong YU. Current Status of Research on mRNA Vaccines Against Viral Infectious Diseases [J]. Current Biotechnology, 2024, 14(4): 566-575. |
| [5] | Hongyan JIAO, Guochao LI, Liang CHANG, Yanyi LI, Lili ZHAI. Overview of Norovirus Vaccine Research [J]. Current Biotechnology, 2024, 14(1): 17-25. |
| [6] | Na LYU, Yanyi LI, Zhuqing MA. Advances on Prevention and Treatment of Human Respiratory Syncytial Virus [J]. Current Biotechnology, 2024, 14(1): 26-34. |
| [7] | Yingying LIU, Kexin ZHAO, Yifei WANG, Kaixuan LU, Kongxin HU. Research Progress of Biological Detection Reagent Preservation Technology [J]. Current Biotechnology, 2024, 14(1): 94-101. |
| [8] | Dan YU, Yunlong MA, Fang WAN, Jianqiang WU. Advances on Research and Application of mRNA Vaccines [J]. Current Biotechnology, 2023, 13(4): 492-498. |
| [9] | Lang GAO, Sixue YU, Chunsen YUAN, Zhiwei SHAN, Pengxiang ZHAO. Research Progress of Mucin in Tumor Immunotherapy [J]. Current Biotechnology, 2023, 13(3): 390-398. |
| [10] | Wengui LI, Yatang CHEN. The Status Progress in the Research of Recombinant Vaccine Based on Canarypox Virus [J]. Current Biotechnology, 2022, 12(1): 36-43. |
| [11] | Dan LI, Haozhi SONG, Weisong GAO, Xingjian LIU, Zhifang ZHANG, Yinyu LI. Prokaryotic Expression and Immunogenicity Detection of the H Protein of Peste des Petits Ruminants Virus [J]. Current Biotechnology, 2021, 11(6): 770-776. |
| [12] | YAO Shunyu, ZHANG Lilin*. Progress on Newcastle Disease Vaccine [J]. Curr. Biotech., 2020, 10(5): 470-478. |
| [13] | SHI Jiangchuan1,2, SHI Jia2*, YU Mingxiao2, ZHAO Weixue2, XU Hao2, XU Lei2. Optimization of Spray Drying Technology for Hemp Seed Oil Microcapsules by Response Surface Methodology [J]. Curr. Biotech., 2020, 10(2): 198-204. |
| [14] | ZHAO Yunxia1, JIANG Kui2*, GUO Aiying1, LI Jingyan1. Preparation of Rabbit Antiserum from Type 1 Pneumonia and its Quality Control in Detection of Polysaccharide Content [J]. Curr. Biotech., 2019, 9(4): 416-421. |
| [15] | LIU Mengyu1, XIE Fei1, ZHANG Xin1, ZHAO Pengxiang1,2*. Review of Immunotherapy for Glioblastoma [J]. Curr. Biotech., 2019, 9(3): 223-230. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||