Current Biotechnology ›› 2024, Vol. 14 ›› Issue (6): 993-1003.DOI: 10.19586/j.2095-2341.2024.0094
• Reviews • Previous Articles Next Articles
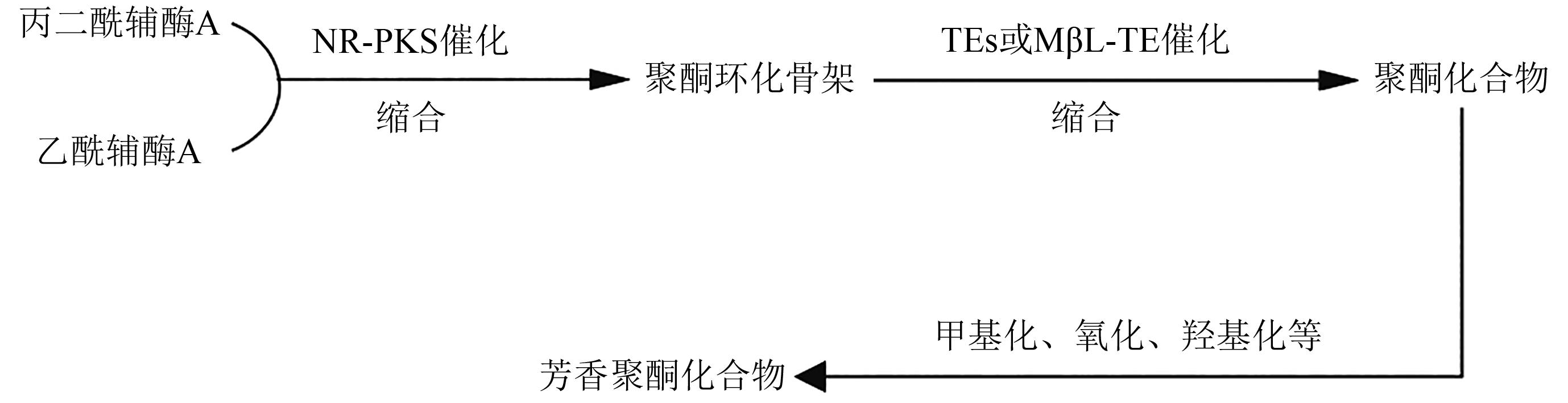
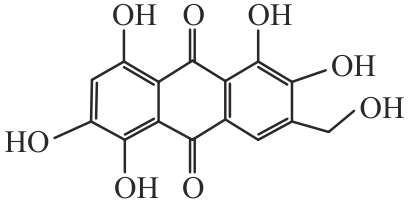
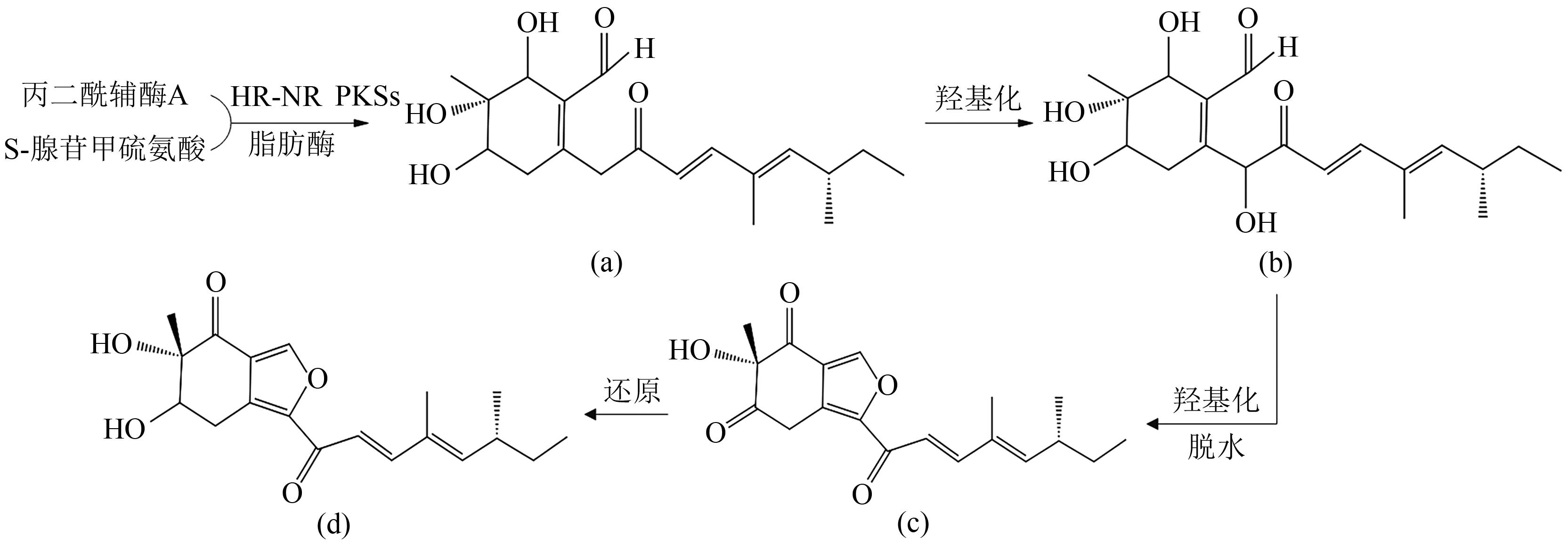
Research Progress on Polyketides from Marine Fungi
Shaoting PAN( ), Boxuan WANG, Jiaxin CHEN, Jiajun CAI, Yanshen LIN, Lingzhi TANG, Xuan HONG(
), Boxuan WANG, Jiaxin CHEN, Jiajun CAI, Yanshen LIN, Lingzhi TANG, Xuan HONG( )
)
- Xiamen Key Laboratory of Marine Medicinal Natural Products Resources,Xiamen Medical College,Fujian Xiamen 361023,China
-
Received:2024-05-06Accepted:2024-08-13Online:2024-11-25Published:2024-12-27 -
Contact:Xuan HONG
海洋真菌来源的聚酮类化合物研究进展
潘少婷( ), 王博轩, 陈佳鑫, 蔡佳君, 林彦伸, 唐灵芝, 洪璇(
), 王博轩, 陈佳鑫, 蔡佳君, 林彦伸, 唐灵芝, 洪璇( )
)
- 厦门医学院,厦门市海洋药用天然产物资源重点实验室,福建 厦门 361023
-
通讯作者:洪璇 -
作者简介:潘少婷 E-mail:1075047301@qq.com; -
基金资助:国家级大学生创新创业训练项目(202312631001);厦门市自然科学基金面上项目(3502Z20227227);福建省自然科学基金面上项目(2022J011401);福建省教育科学“十四五”规划课题(FJJKBK22-049)
CLC Number:
Cite this article
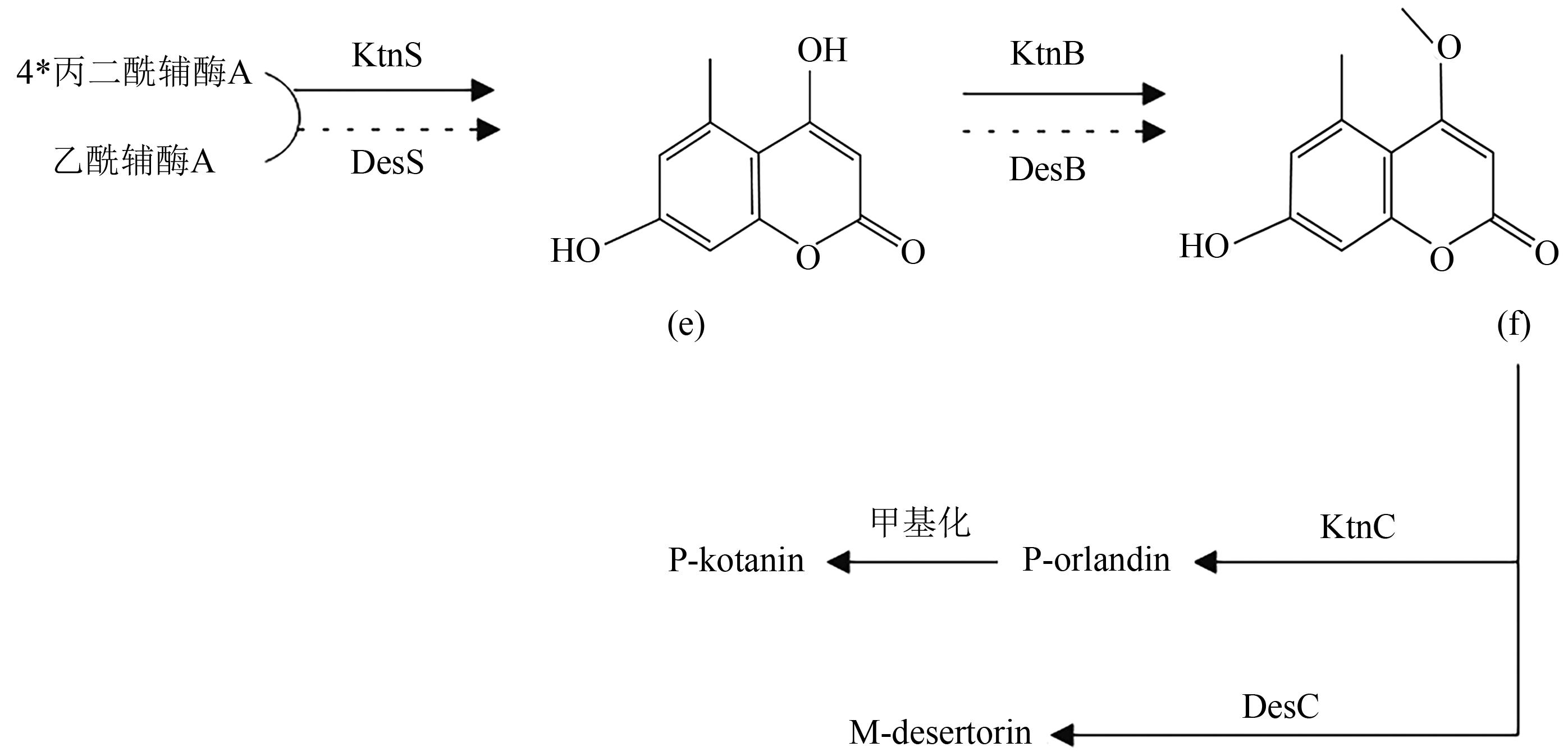
Shaoting PAN, Boxuan WANG, Jiaxin CHEN, Jiajun CAI, Yanshen LIN, Lingzhi TANG, Xuan HONG. Research Progress on Polyketides from Marine Fungi[J]. Current Biotechnology, 2024, 14(6): 993-1003.
潘少婷, 王博轩, 陈佳鑫, 蔡佳君, 林彦伸, 唐灵芝, 洪璇. 海洋真菌来源的聚酮类化合物研究进展[J]. 生物技术进展, 2024, 14(6): 993-1003.
share this article
| 来源 | 生物合成关键酶 | 产物结构 | 生物活性 | 文献 |
|---|---|---|---|---|
| Aspergillus nidulans | AptA、结合Mn2+的AptB以及黄素单加氧酶AptC | 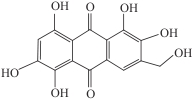 | 生姜青枯病(姜瘟病)的防治 | [ |
| Asperfuranone | HR-PKSs(AteafoE)、NR-PKSs(AteafoD)以及脂肪酶 | 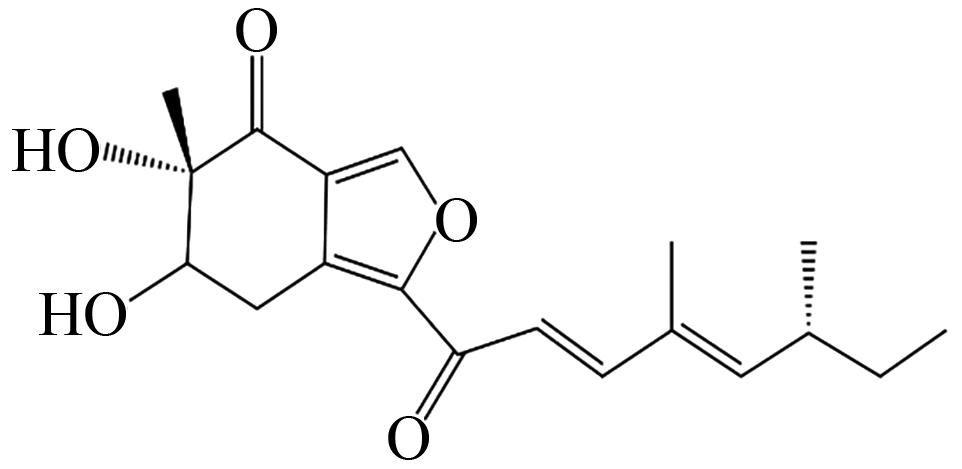 | 抗菌、抗氧化 | [ |
| Aspergillus niger | HR-PKSs、O-甲基转移酶(KtnB) | 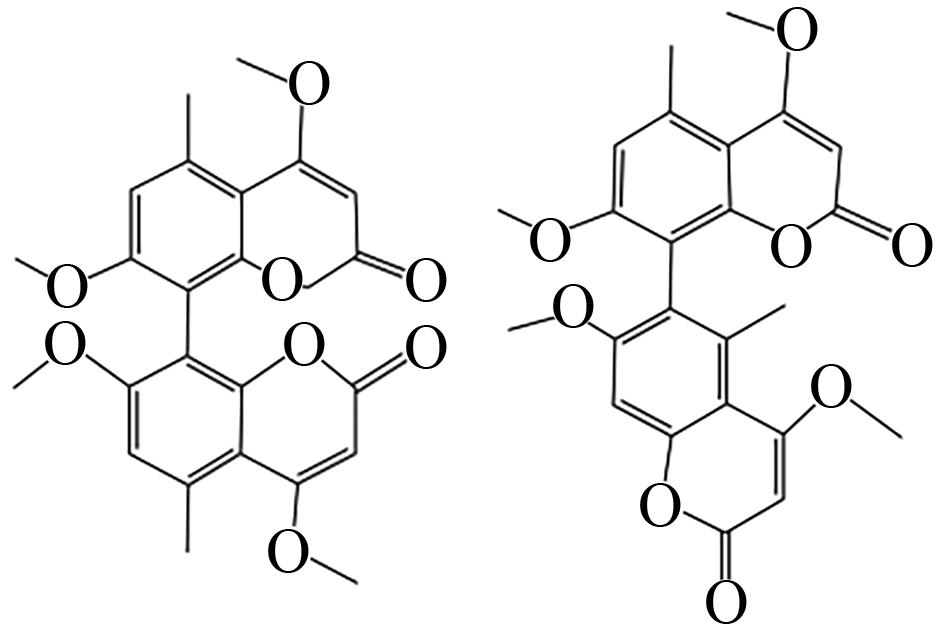 | - | [ |
| Aspergillus fumigatus | PKS-NRPSs、甲基转移酶 | 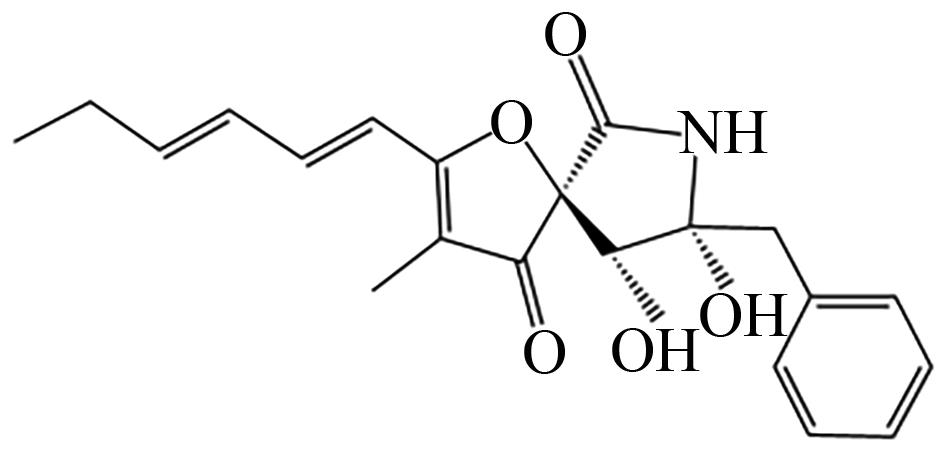 | - | [ |
| A.niger | AdaA、甲基转移酶(AdaD) | 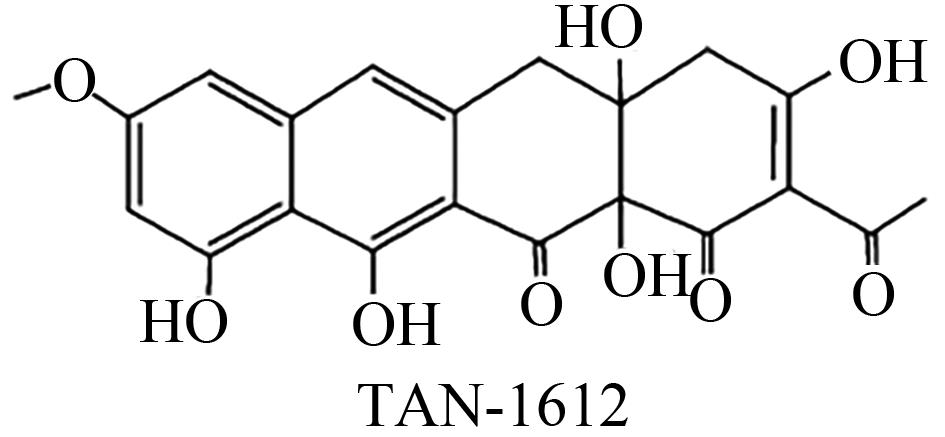 | 受体拮抗剂 | [ |
| Cladosporium fulvum | 细胞色素P450酶、还原酶ClaC、脱水酶ClaB、脱羧酶ClaH | 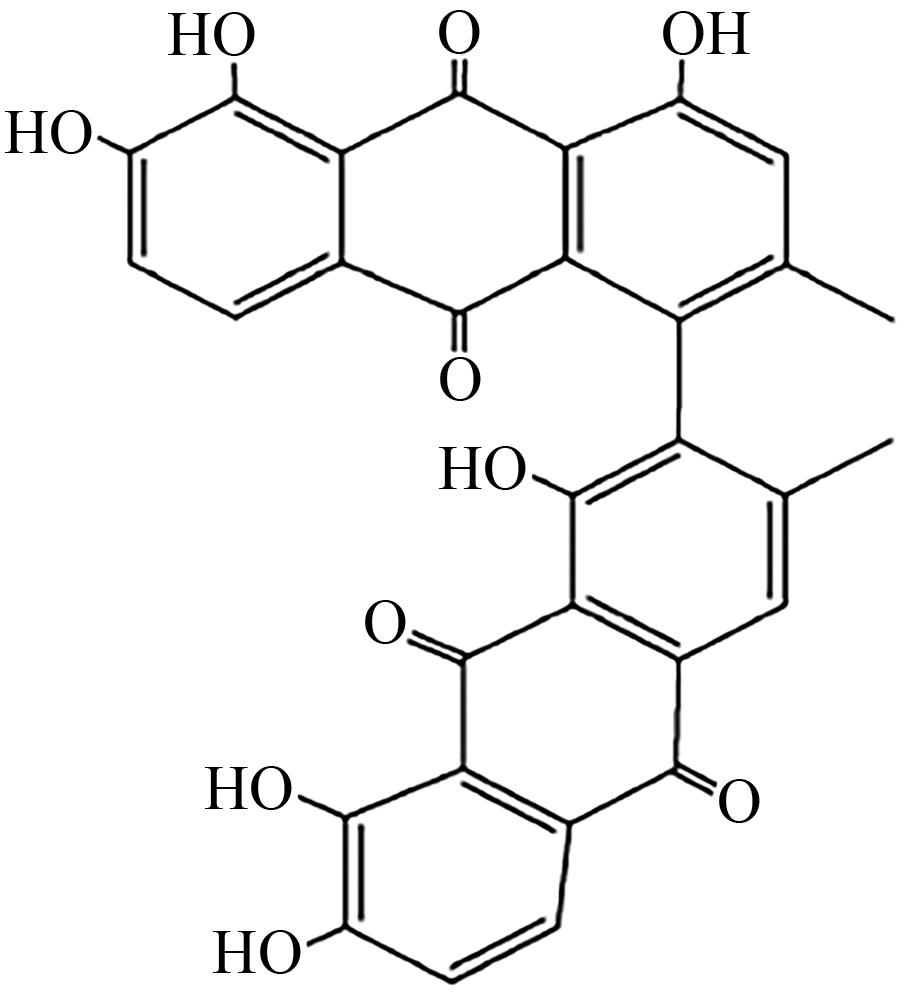 | 细胞毒性 | [ |
| F.fujikuroi | O-甲基转移酶Fsr2 | 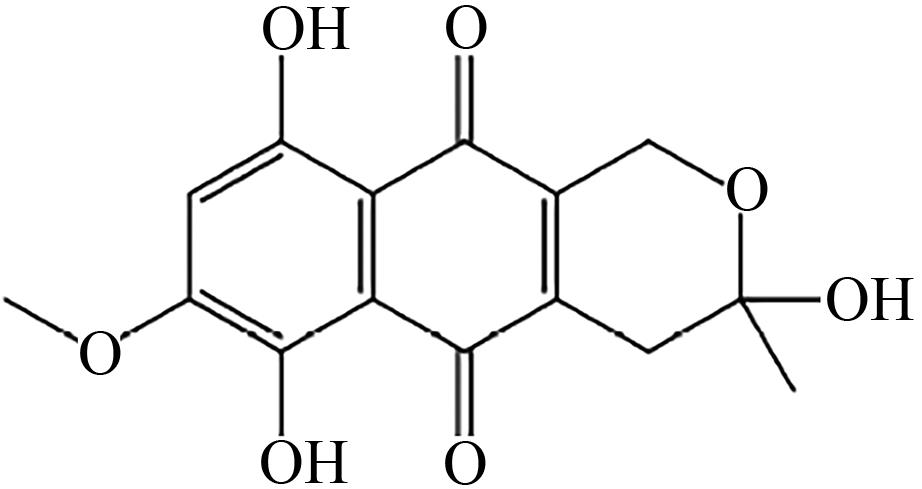 | - | [ |
| A. nidulans | SlACAS | 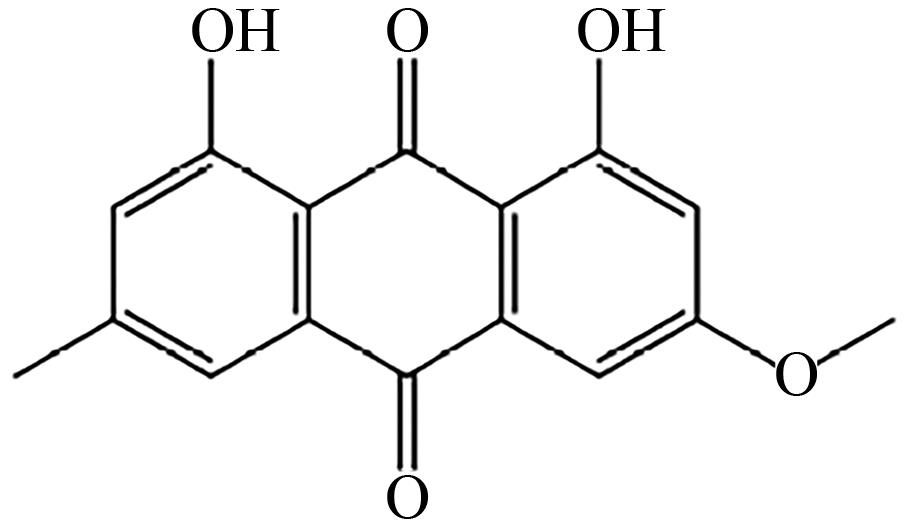 | 抗氧化剂 | [ |
Table 1 Progress on the biosynthetic pathways of marine fungal polyketides
| 来源 | 生物合成关键酶 | 产物结构 | 生物活性 | 文献 |
|---|---|---|---|---|
| Aspergillus nidulans | AptA、结合Mn2+的AptB以及黄素单加氧酶AptC |  | 生姜青枯病(姜瘟病)的防治 | [ |
| Asperfuranone | HR-PKSs(AteafoE)、NR-PKSs(AteafoD)以及脂肪酶 |  | 抗菌、抗氧化 | [ |
| Aspergillus niger | HR-PKSs、O-甲基转移酶(KtnB) |  | - | [ |
| Aspergillus fumigatus | PKS-NRPSs、甲基转移酶 |  | - | [ |
| A.niger | AdaA、甲基转移酶(AdaD) |  | 受体拮抗剂 | [ |
| Cladosporium fulvum | 细胞色素P450酶、还原酶ClaC、脱水酶ClaB、脱羧酶ClaH |  | 细胞毒性 | [ |
| F.fujikuroi | O-甲基转移酶Fsr2 |  | - | [ |
| A. nidulans | SlACAS |  | 抗氧化剂 | [ |
| 1 | 江德鲁. 两类聚酮天然抗生素的全合成研究[D]. 上海: 华东师范大学, 2020. |
| JIANG D L. Synthetic studies towards the total synthesis of two types of polvketide natural antibiotics[D]. Shanghai: East China Normal University, 2020. | |
| 2 | 李雪清,于大永,冯宝民,等.微生物代谢产物中聚酮类化合物的研究进展[J].中成药,2016,38(10):2233-2239. |
| LI X Q, YU D Y, FENG B M, et al.. Research progress of polyketones in microbial metabolites[J]. Chin. Tradit. Pat. Med., 2016, 38(10): 2233-2239. | |
| 3 | 胡春生,吴祖泽,张庆林.聚酮类化合物异源表达研究进展[J].生物技术通讯,2013,24(4):573-578. |
| HU C S, WU Z Z, ZHANG Q L. Research progress in heterologous express of polyketides[J]. Lett. Biotechnol., 2013, 24(4): 573-578. | |
| 4 | 张柳红, 宋双. 海洋来源的聚酮类化合物及其活性研究进展[J] 中山大学研究生学刊(自然科学·医学版), 2015, 36(4):25-34. |
| ZHANG L H, SONG S. Research progress on polyketides derived from marine sources and their activities[J]. J. Graduates Sun YAT-SEN Univ.(Nat. Sci. Med.), 2015, 36(4): 25-34. | |
| 5 | 牟鹏云. 两株海洋真菌次级代谢产物分离鉴定及抑菌活性研究[D]. 新疆阿拉尔:塔里木大学, 2021. |
| MOU P Y. Isolation, identification and antibacterial activity of secondary metabolites from two marine fungi[D]. Xinjiang Alaer: Tarim University, 2021. | |
| 6 | 华熠.共生曲霉菌D来源的聚酮类化合物及其生物合成初探[D].杭州:浙江工业大学,2019. |
| 7 | 汪淑文. 聚酮类化合物红霉素及抗霉素的碳骨架改造研究[D]. 武汉:武汉大学, 2017. |
| WANG S W. Study on Engineering Erythromycin and Antimycin Polyketide Carbon Scaffold[D]. Wuhan: Wuhan University, 2017. | |
| 8 | LEI H, LEI J, ZHOU X, et al.. Cytotoxic polyketides from the marine sponge-derived fungus Pestalotiopsis heterocornis XWS 03F09[J/OL]. Mol. Basel Switz., 2019, 24(14): E2655[2024-07-25]. . |
| 9 | 胡彩云, 李赛妮, 陈玉婵,等. 深海真菌Diaporthe phaseolorum FS459的聚酮类化合物研究[J] 有机化学, 2021, 41(4): 1591-1598. |
| HU C Y, LI S N, CHEN Y C,et al.. Polyketides from the deep-sea-derived fungus Diaporthe phaseolorum FS459[J]. Chin. J. Organic Chem. 2021, 41(4):1591-1598. | |
| 10 | WLODEK A, KENDREW S G, COATES N J, et al.. Diversity oriented biosynthesis via accelerated evolution of modular gene clusters[J/OL]. Nat. Commun., 2017, 8: 1206[2024-07-25]. . |
| 11 | COZE F, GILARD F, TCHERKEZ G, et al.. Carbon-flux distribution within Streptomyces coelicolor metabolism: a comparison between the actinorhodin-producing strain M145 and its non-producing derivative M1146[J/OL]. PLoS One, 2013, 8(12): e84151[2024-07-25]. . |
| 12 | 刘璐. 一株海洋来源链格孢聚酮合酶及真菌非还原型聚酮合酶PT结构域系统研究[D]. 山东青岛: 中国海洋大学, 2015. |
| LIU L. Systematic analyses of polyketide synthases from a marine derived alternaria alternata and fungal NR-PKS product template domains[D]. Shandong Qingdao: Ocean University of China, 2015. | |
| 13 | 万影. 链格孢菌中聚酮合酶的挖掘及P450酶HqlC的催化活性研究[D]. 长沙: 中南大学, 2022. |
| WAN Y. Mining of polyketide synthases in Alternaria alternata and biocatalytic study of the cytochrome P450 enzyme HqlC[D]. Changsha:Central South University, 2022. | |
| 14 | 杨晓钰,何佳宁,牛雪梅.真菌中PKS-NRPS杂合天然产物研究进展[J].中国科学(生命科学),2019,49(7):848-864. |
| YANG X Y, HE J N, NIU X M. Research progress on fungal PKS-NRPS hybrid metabolites[J]. Sci. Sin. Vitae, 2019, 49(7): 848-864. | |
| 15 | KORMAN T P, CRAWFORD J M, LABONTE J W, et al.. Structure and function of an iterative polyketide synthase thioesterase domain catalyzing Claisen cyclization in aflatoxin biosynthesis[J]. Proc. Natl. Acad. Sci. USA, 2010, 107(14): 6246-6251. |
| 16 | 饶海密,梁冬梅,李伟国,等.真菌芳香聚酮化合物的合成生物学研究进展[J].中国生物工程杂志,2020,40(9):52-61. |
| RAO H M, LIANG D M, LI W G, et al.. Advances in synthetic biology of fungal aromatic polyketides[J]. China Biotechnol., 2020, 40(9): 52-61. | |
| 17 | LI Y, HCHOOI Y, SHENG Y, et al.. Comparative characterization of fungal anthracenone and naphthacenedione biosynthetic pathways reveals an α-hydroxylation-dependent claisen-like cyclization catalyzed by a dimanganese thioesterase[J]. J. Am. Chem. Soc., 2011, 133(39): 15773-15785. |
| 18 | 陈锡玮,许蒙,冯程,等.真菌聚酮化合物生物合成研究进展[J].生物工程学报,2018,34(2):151-164. |
| CHEN X W, XU M, FENG C, et al.. Progress in fungal polyketide biosynthesis[J]. Chin. J. Biotechnol., 2018, 34(2): 151-164. | |
| 19 | CHIANG Y M, OAKLEY C E, AHUJA M, et al.. An efficient system for heterologous expression of secondary metabolite genes in Aspergillus nidulans [J]. J. Am. Chem. Soc., 2013, 135(20): 7720-7731. |
| 20 | TAKINO J, KOTANI A, OZAKI T, et al.. Biochemistry-guided prediction of the absolute configuration of fungal reduced polyketides[J]. Angew. Chem. Int. Ed Engl., 2021, 60(43): 23403-23411. |
| 21 | STOTHERS J B, STOESSL A. Confirmation of the polyketide origin of kotanin by incorporation of [1,2-13C2]acetate[J]. Can. J. Chem., 1988, 66(11):2816-2818. |
| 22 | HÜTTEL W, MÜLLER M. Regio- and stereoselective intermolecular oxidative phenol coupling in kotanin biosynthesis by Aspergillus niger [J]. ChemBioChem, 2007, 8(5): 521-529. |
| 23 | MAZZAFERRO L S, HÜTTEL W, FRIES A, et al.. Cytochrome P450-catalyzed regio- and stereoselective phenol coupling of fungal natural products[J]. J. Am. Chem. Soc., 2015, 137(38): 12289-12295. |
| 24 | 滕安然, 李力, 苏红梅 聚酮合酶和非核糖体多肽合成酶杂合体研究进展 [J] 药物生物技术, 2019, 26(5):455-460. |
| TENG A R, LI L, SU H M. The research progress in NRPS and PKS hybrid metabolites[J]. Pharm. Biotechnol., 2019, 26(5): 455-460. | |
| 25 | BOETTGER D, HERTWECK C. Molecular diversity sculpted by fungal PKS-NRPS hybrids[J]. ChemBioChem, 2013, 14(1): 28-42. |
| 26 | MAIYA S, GRUNDMANN A, LI X, et al.. Identification of a hybrid PKS/NRPS required for pseurotin A biosynthesis in the human pathogen Aspergillus fumigatus [J]. ChemBioChem, 2007, 8(14): 1736-1743. |
| 27 | TSUNEMATSU Y, FUKUTOMI M, SARUWATARI T, et al.. Elucidation of pseurotin biosynthetic pathway points to trans-acting C-methyltransferase: generation of chemical diversity[J]. Angew. Chem. Int. Ed. Engl., 2014, 53(32): 8475-8479. |
| 28 | WIEMANN P, GUO C J, PALMER J M, et al.. Prototype of an intertwined secondary-metabolite supercluster[J]. Proc. Natl. Acad. Sci. USA, 2013, 110(42): 17065-17070. |
| 29 | ZOU Y, XU W, TSUNEMATSU Y, et al.. Methylation-dependent acyl transfer between polyketide synthase and nonribosomal peptide synthetase modules in fungal natural product biosynthesis[J]. Org. Lett., 2014, 16(24): 6390-6393. |
| 30 | SCOTT G, H M C, BENEDETTA S, et al.. Elucidation of cladofulvin biosynthesis reveals a cytochrome P450 monooxygenase required for anthraquinone dimerization[J]. Proc. Natl. Acad. Sci. USA, 2016, 113(25): 6851-6856. |
| 31 | STUDT L, WIEMANN P, KLEIGREWE K, et al.. Biosynthesis of fusarubins accounts for pigmentation of Fusarium fujikuroi perithecia[J]. Appl. Environ. Microbiol., 2012, 78(12): 4468-4480. |
| 32 | FRANCO M E, LÓPEZ S, MEDINA R, et al.. Draft genome sequence and gene annotation of Stemphylium lycopersici strain CIDEFI-216[J]. Genome Announc., 2015, 3(5): e01069-15. |
| 33 | 刘述春,孙炳达,旺姆,等.一株毛壳霉属真菌中新结构活性聚酮类化合物研究[J].菌物学报,2010,29(5):726-731. |
| LIU S C, SUN B D, WANG M, et al.. Chaetomones A-E, new bioactive polyketides from Chaetomium sp.[J]. Mycosystema, 2010, 29(5): 726-731. | |
| 34 | 马忠莲, 姚光山, 王长云. 海洋真菌Metarhizium sp. P2100来源的α-吡喃酮类化合物研究[J] 中国海洋药物, 2023, 42(1):12-18. |
| MA Z L, YAO G S, WANG Z Y. Study on a- pyrone derivatives from marine- derived fungus Metarhizium sp. P2100[J]. Chin. J. Marine Drugs, 2023, 42(1): 12-18. | |
| 35 | 王荫荫,王苗,钱声艳,等.链霉菌Streptomyces sp.FJS31-2产卤化二型聚酮类化合物的发酵条件优化[J].中国酿造,2017,36(1):66-69. |
| WANG Y Y, WANG M, QIAN S Y, et al.. Optimization of fermentation conditions for halogenated type Ⅱ polyketides production from Streptomyces sp. FJS31-2[J]. China Brew., 2017, 36(1): 66-69. | |
| 36 | 郭佳.基于聚酮合酶基因挖掘微生物代谢产物中新型聚酮化合物的研究[D].南京:南京农业大学,2018. |
| 37 | SUN K, ZHU G, HAO J, et al.. Chemical-epigenetic method to enhance the chemodiversity of the marine algicolous fungus, Aspergillus terreus OUCMDZ-2739[J]. Tetrahedron, 2018, 74(1): 83-87. |
| 38 | LIU W, WANG L, WANG B, et al.. Diketopiperazine and diphenylether derivatives from marine algae-derived Aspergillus versicolor OUCMDZ-2738 by epigenetic activation[J/OL]. Mar Drugs, 2018, 17(1): E6[2024-07-25]. . |
| 39 | 方岫琴, 王文璟, 李华东,等. 微生物次级代谢产物多样性发掘方法 [J] 中国抗生素杂志, 2024, 49(4): 415-426. |
| FANG X Q, WANG W J, LI H D, et al.. Approaches for investigating the variety of microbial secondary metabolites[J]. Chin. J. Antibiot., 2024, 49(4): 415-426. | |
| 40 | 丛梦静,胡怡伟,赵凯,等.深海冷泉来源真菌Talaromyces helicus SCSIO41311中聚酮类化学成分研究[J].热带海洋学报,2022,41(5):117-120. |
| CONG M J, HU Y W, ZHAO K, et al.. Study on the polyketides from a cold-seep-derived Talaromyces helicus SCSIO41311[J]. J. Trop. Oceanogr., 2022, 41(5): 117-120. | |
| 41 | 刘向红. 四株海洋藻栖真菌次生代谢产物的结构与活性研究[D]. 山东烟台:中国科学院大学(中国科学院烟台海岸带研究所), 2022. |
| LIU X H. Structures and activities of secondary metabolites from four marine algicolous fungi[D]. Shandong Yantai: University of Chinese Academy of Sciences(Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences), 2022. | |
| 42 | 周晓雪, 杨佳凡, 李明哲,等. 珊瑚来源真菌Parengyodontium album SCSIO SX7W11中聚酮化合物的发现及其生物合成途径分析[J] 微生物学报, 2021, 61(11): 3631-3641. |
| ZHOU X X, YANG J F, LI M Z, et al.. Discovery of polyketide natural products from corals derived fungi Parengyodontium album SCSIO SX7W11 and their biosynthetic pathway analysis[J]. Acta Microbiol. Sin., 2021, 61(11):3631-3641. | |
| 43 | 郭珩.深海真菌Diaporthe phaseolorum次级代谢产物及其生物活性研究[D].广州:广东药科大学,2019. |
| 44 | 吴金涛.两株南海红树林根际土壤真菌的聚酮类次级代谢产物研究[D].扬州:扬州大学,2021. |
| 45 | LI M Q, HUANG H B, CHEN S N,et al.. Polyketides from the deep-sea-derived fungus Talaromyces indigoticus FS688 and their cytotoxicites[J]. Chin. J. Organic Chem., 2022, 42(9):2975-2980. |
| 46 | 冉燕琴.三株海洋真菌次级代谢产物的研究[D].广州:广东药科大学,2020. |
| 47 | LI S W, CUI W X, HUAN X J, et al.. A new bis-γ-pyrone polypropionate of onchidiol family from marine pulmonate mollusk Onchidium sp.[J]. Nat. Prod. Res., 2020, 34(14): 1971-1976. |
| 48 | XU Y, ZHANG M, LIU Q-A, et al.. New verrucosidin derivatives from the marine-derived fungus Penicillium sp. XL-01[J/OL]. Nat. Prod. Commun., 2018, 13(10)1934578X1801301024[2024- 07-25].. |
| 49 | YANG T, YAMADA K, ZHOU T, et al.. Akazamicin, a cytotoxic aromatic polyketide from marine-derived Nonomuraea sp.[J]. J. Antibiot., 2019, 72: 202-209. |
| 50 | 章宝丹, 任晋玮, 翟亚楠,等. 1株海洋真菌Penicillium sp.的次级代谢产物及抗细菌活性研究[J]. 中国抗生素杂志, 2024, 49(1): 35-40. |
| ZHANG B D, REN J W, ZHAI Y N, et al.. The secondary metabolites isolated from the marine-derived fungus Penicillium sp. and their antibacterial activities[J]. Chin. J. Antibiotics, 2024, 49(1): 35-40. | |
| 51 | LIN C, HUANG R, LIU J, et al.. Antibacterial polyketides isolated from the marine-derived fungus Fusarium solani 8388[J/OL]. J. Fungi Basel Switz., 2023, 9(9): 875[2024-07-25]. . |
| 52 | LIU W H, DING Y, JI X, et al.. Curvulaide A, a bicyclic polyketide with anti-anaerobic bacteria activity from marine-derived Curvularia sp.[J]. J. Antibiot., 2019, 72: 111-113. |
| 53 | CAI C, CHEN Y, ZHOU L, et al.. Antimicrobial polyketides from the marine-derived fungus Spiromastix sp. SCSIO F190[J]. J. Nat. Prod., 2023, 86(3): 589-595. |
| 54 | 张连庆.三株海洋来源真菌次级代谢产物及其活性多样性的研究[D].青岛:中国海洋大学,2014. |
| 55 | QIN C, LIN X, LU X, et al.. Sesquiterpenoids and xanthones derivatives produced by sponge-derived fungus Stachybotry sp. HH1 ZSDS1F1-2[J]. J. Antibiot., 2015, 68(2): 121-125. |
| 56 | CHEN W, ZHANG J, QI X, et al.. P-terphenyls as anti-HSV-1/2 agents from a deep-sea-derived Penicillium sp.[J]. J. Nat. Prod., 2021, 84(11): 2822-2831. |
| 57 | ABDELWAHAB G M, MIRA A, CHENG Y B, et al.. Acetylcholine esterase inhibitory activity of green synthesized nanosilver by naphthopyrones isolated from marine-derived Aspergillus niger [J/OL]. PLoS One, 2021, 16(9): e0257071[2024-07-25]. . |
| 58 | HAN W, CAI J, ZHONG W, et al.. Protein tyrosine phosphatase 1B (PTP1B) inhibitorsfrom the deep-sea fungus Penicillium chrysogenum SCSIO 07007[J/OL]. Bioorg. Chem., 2020, 96: 103646[2024-07-25]. . |
| 59 | WU Z H, LIU D, XU Y, et al.. Antioxidant xanthones and anthraquinones isolated from a marine-derived fungus Aspergillus versicolor [J]. Chin. J. Nat. Med., 2018, 16(3): 219-224. |
| 60 | LIU D S, RONG X G, KANG H H, et al.. Raistrickiones A-E from a highly productive strain of Penicillium raistrickii generated through thermo change[J/OL]. Mar. Drugs, 2018, 16(6): E213[2024-07-25]. . |
| 61 | LIU Z, FRANK M, YU X, et al.. Secondary metabolites from marine-derived fungi from China[J]. Prog. Chem. Org. Nat. Prod., 2020, 111: 81-153. |
| [1] | Lin XIA, Xiangli XU, Xueyun WANG, Jun YANG, Mingzhu WU, Weiwu SONG. Research Progress on the Biosynthesis of Chlorogenic Acid in Plant [J]. Current Biotechnology, 2024, 14(6): 973-979. |
| [2] | Qiaoli CHEN, Jie HUANG, Senyu CHEN, Shaoting PAN, Lingzhi TANG, Xuan HONG. Research Progress on Secondary Metabolites of Marine Streptomyces [J]. Current Biotechnology, 2023, 13(6): 844-852. |
| [3] | Yanjiao LI, Yuan GAO, Lei WANG, Lan ZHANG. Research Progress of Tocotrienol [J]. Current Biotechnology, 2021, 11(6): 668-675. |
| [4] | YAO Xinglan, WANG Lei*, ZHANG Lan*. Progress of Vitamin E Biofortification in Plants [J]. Curr. Biotech., 2020, 10(5): 479-486. |
| [5] | DONG Luna, CAO Hao, ZHANG Xinyu, WANG Haisheng*. Research Progress on Dihydroquercetin [J]. Curr. Biotech., 2020, 10(3): 226-233. |
| [6] | LI Meng-ying, YAN Pei-sheng*, GAO Xiu-jun, SUN Xiao-lei. Progress on Diversity and Secondary Metabolites Biological Activity of Deep-sea Fungi [J]. Curr. Biotech., 2015, 5(3): 170-175. |
| [7] | WANG Quan-fu, MIAO Miao, HOU Yan-hua*, SHI Yong-lei, HAN Han, WU Ying-ying, YAN Tian-qi, Qu Jun-jie. Progress on Thioredoxin [J]. Curr. Biotech., 2015, 5(3): 196-200. |
| [8] | WANG Xiao-jing1, LU Wei1, XU Yu-quan1*, LIANG Xiao-dong2. Progress on the Biosynthesis of Fungal Benzenediol Lactone Polyketides [J]. Curr. Biotech., 2015, 5(2): 89-94. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||