Current Biotechnology ›› 2023, Vol. 13 ›› Issue (5): 698-703.DOI: 10.19586/j.2095-2341.2023.0064
• Reviews • Previous Articles Next Articles
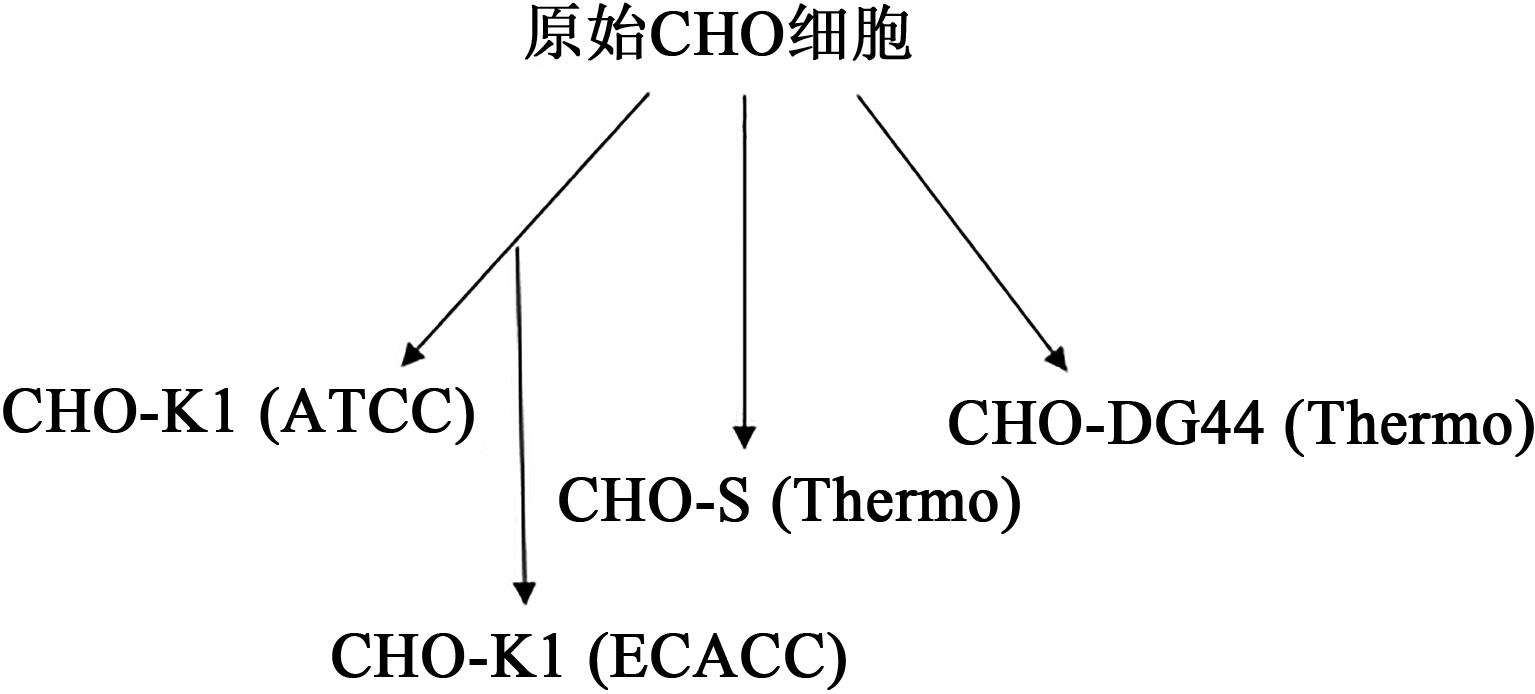
Effects of Different Sources of CHO Host Cells on Antibody Expression
Hui CAO( ), Jing DONG, Yu JIA, Yifan JIANG(
), Jing DONG, Yu JIA, Yifan JIANG( )
)
- State Key Laboratory of Antibody Drug Development,Hebei Engineering Research Center of Antibody Medicine,New Drug Research and Development Co. Ltd,North China Pharmaceutical Corporation,Shijiazhuang 050015,China
-
Received:2023-05-06Accepted:2023-06-07Online:2023-09-25Published:2023-10-10 -
Contact:Yifan JIANG
不同亚型CHO宿主细胞对抗体表达的影响
- 华北制药集团新药研究开发有限责任公司,抗体药物河北省工程研究中心,抗体药物研究国家重点实验室,石家庄 050015
-
通讯作者:江一帆 -
作者简介:曹辉 E-mail: 610753996@qq.com; -
基金资助:河北省科技重大专项(21282401Z)
CLC Number:
Cite this article
Hui CAO, Jing DONG, Yu JIA, Yifan JIANG. Effects of Different Sources of CHO Host Cells on Antibody Expression[J]. Current Biotechnology, 2023, 13(5): 698-703.
曹辉, 董静, 贾宇, 江一帆. 不同亚型CHO宿主细胞对抗体表达的影响[J]. 生物技术进展, 2023, 13(5): 698-703.
share this article
| 1 | KAPLON H, CHENOWETH A, CRESCIOLI S. Antibodies to watch in 2023[J/OL]. mAbs,2023, 15(1): e2153410[2022-12-06]. . |
| 2 | 药智网.2021全球药品销售TOP10: 药王“修美乐”跌落榜首,新冠疫苗大卖 367亿[EB/OL]. [2023-05-30]. . |
| 3 | 郑惠惠, 江洪. CHO细胞表达系统研究进展[J]. 生物技术进展, 2016, 6(4): 239-243. |
| 4 | 陶维红, 秦民民, 张哲如. CHO细胞株开发技术策略探讨[J]. 生物技术进展, 2014, 4(6): 394-399. |
| 5 | LAI T, YANG Y, NG S K. Advances in mammalian cell line development technologies for recombinant protein production[J]. Pharmaceuticals, 2013, 6(5): 579-603. |
| 6 | FISCHER S, HANDRICK R, OTTE K. The art of CHO cell engineering: a comprehensive retrospect and future perspectives[J]. Biotechnol. Adv., 2015, 33(8): 1878-1896. |
| 7 | WEINGUNY M, KLANERT G, EISENHUT P, et al.. Directed evolution approach to enhance efficiency and speed of outgrowth during single cell subcloning of Chinese Hamster Ovary cells[J]. Comput. Struct. Biotechnol. J., 2020, 18: 1320-1329. |
| 8 | PUCK T T, CIECIURA S J, ROBINSON A. Genetics of somatic mammalian cells. Ⅲ. Long-term cultivation of euploid cells from human and animal subjects[J]. J. Exp. Med., 1958, 108(6): 945-956. |
| 9 | TIHANYI B, NYITRAY L. Recent advances in CHO cell line development for recombinant protein production[J]. Drug Discov. Today Technol., 2020, 38: 25-34. |
| 10 | WURM F. CHO quasispecies-mplications for manufacturing processes[J]. Processes, 2013, 1(3): 296-311. |
| 11 | LEWIS N E, LIU X, LI Y, et al.. Genomic landscapes of Chinese hamster ovary cell lines as revealed by the Cricetulus griseus draft genome[J]. Nat. Biotechnol., 2013, 31(8): 759-765. |
| 12 | HUHN S, CHANG M, KUMAR A, et al.. Chromosomal instability drives convergent and divergent evolution toward advantageous inherited traits in mammalian CHO bioproduction lineages[J/OL]. iScience, 2022, 25(4): 104074[2023-01-15]. . |
| 13 | PASSERINI V, OZERI-GALAI E, DE PAGTER M S, et al.. The presence of extra chromosomes leads to genomic instability[J/OL]. Nat. Commun., 2016, 7: 10754[2023-01-15]. . |
| 14 | DREWS R M, HERNANDO B, TARABICHI M, et al.. A pan-cancer compendium of chromosomal instability[J]. Nature, 2022, 606(7916): 976-983. |
| 15 | TAKADA H, MIURA T, FUJIBAYASHI S, et al.. Detailed chromosome analysis of wild-type, immortalized fibroblasts with SV40T, E6E7, combinational introduction of cyclin dependent kinase 4, cyclin D1, telomerase reverse transcriptase[J]. In Vitro Cell. Dev. Biol. Anim., 2021, 57(10): 998-1005. |
| 16 | TURILOVA V I, GORYACHAYA T S, YAKOVLEVA T K. Chinese hamster ovary cell line DXB-11: chromosomal instability and karyotype heterogeneity[J/OL]. Mol. Cytogenet., 2021, 14(1): 11[2023-03-24]. . |
| 17 | RAY M, MOHANDAS T. Proposed banding nomenclature for the Chinese hamster chromosomes (Cricetulus griseus)[J]. Cytogenet. Cell Genet., 1976, 16(1-5): 83-91. |
| 18 | YAMANO N, KUMAMOTO T, TAKAHASHI M, et al.. Stability difference of each chromosome in Chinese Hamster Ovary cell line[J]. BMC Proc., 2015, 9(9): P1 [2023-03-08]. . |
| 19 | VCELAR S, MELCHER M, AUER N, et al.. Changes in chromosome counts and patterns in CHO cell lines upon generation of recombinant cell lines and subcloning[J/OL]. Biotechnol. J., 2018, 13(3): e1700495[2023-03-08]. . |
| 20 | DEROUAZI M, MARTINET D, BESUCHET SCHMUTZ N, et al.. Genetic characterization of CHO production host DG44 and derivative recombinant cell lines[J]. Biochem. Biophys. Res. Commun., 2006, 340(4): 1069-1077. |
| 21 | OGATA N, NISHIMURA A, MATSUDA T, et al.. Single-cell transcriptome analyses reveal heterogeneity in suspension cultures and clonal markers of CHO-K1 cells[J]. Biotechnol. Bioeng., 2021, 118(2): 944-951. |
| 22 | BAEZ A, SHILOACH J. Effect of elevated oxygen concentration on bacteria, yeasts, and cells propagated for production of biological compounds[J/OL]. Microb. Cell Fact., 2014, 13: 181[2023-03-06]. . |
| 23 | LIN K W, YAN J. Endings in the middle: current knowledge of interstitial telomeric sequences[J]. Mutat. Res., 2008, 658(1-2): 95-110. |
| 24 | SCARCELLI J J, HONE M, BEAL K, et al.. Analytical subcloning of a clonal cell line demonstrates cellular heterogeneity that does not impact process consistency or robustness[J]. Biotechnol. Prog., 2018, 34(3): 602-612. |
| 25 | ARBEITHUBER B, HESTER J, CREMONA M A, et al.. Age-related accumulation of de novo mitochondrial mutations in mammalian oocytes and somatic tissues[J/OL]. PLoS Biol., 2020, 18(7): e3000745[2023-02-14]. . |
| 26 | SOHN S H, CHO E J, JANG I S. Cytogenetic characteristics of Chinese Hamster ovarian cell CHO-K1[J]. Reprod Dev Biol.,2006, 30: 263-270. |
| 27 | VCELAR S, JADHAV V, MELCHER M, et al.. Karyotype variation of CHO host cell lines over time in culture characterized by chromosome counting and chromosome painting[J]. Biotechnol. Bioeng., 2018, 115(1): 165-173. |
| 28 | SRIRANGAN K, LOIGNON M, DUROCHER Y. The use of site-specific recombination and cassette exchange technologies for monoclonal antibody production in Chinese Hamster Ovary cells: retrospective analysis and future directions[J]. Crit. Rev. Biotechnol., 2020, 40(6): 833-851. |
| 29 | WURM F, WURM M. Cloning of CHO cells, productivity and genetic stability-discussion[J/OL]. Processes, 2017, 5(2): 20[2023-03-29]. . |
| 30 | KIMURA S, OMASA T. Genome sequence comparison between Chinese Hamster Ovary (CHO) DG44 cells and mouse using end sequences of CHO BAC clones based on BAC-FISH results[J]. Cytotechnology, 2018, 70(5): 1399-1407. |
| 31 | SOMMEREGGER W, GILI A, STEROVSKY T, et al.. Powerful expression in Chinese Hamster Ovary cells using bacterial artificial chromosomes: parameters influencing productivity[J]. BMC Proc., 2013, 7(6): 1-2. |
| 32 | XU N, MA C, OU J, et al.. Comparative proteomic analysis of three Chinese Hamster Ovary (CHO) host cells[J]. Biochem. Eng. J., 2017, 124: 122-129. |
| 33 | PECCI A, MA X, SAVOIA A, et al.. MYH9: Structure, functions and role of non-muscle myosin ⅡA in human disease[J]. Gene, 2018, 664: 152-167. |
| 34 | REINHART D, DAMJANOVIC L, KAISERMAYER C, et al.. Bioprocessing of recombinant CHO-K 1, CHO-DG44, and CHO-S: CHO expression hosts favor either MAb production or biomass synthesis[J/OL]. Biotechnol. J., 2019, 14(3): e1700686[2023-02-16]. . |
| 35 | VALLA M, OPDAHL S, YTTERHUS B, et al.. DTX3 copy number increase in breast cancer: a study of associations to molecular subtype, proliferation and prognosis[J]. Breast Cancer Res. Treat., 2021, 187(1): 57-67. |
| 36 | HU Z, GUO D, YIP S S M, et al.. Chinese Hamster Ovary K1 host cell enables stable cell line development for antibody molecules which are difficult to express in DUXB11-derived dihydrofolate reductase deficient host cell[J]. Biotechnol. Prog., 2013, 29(4): 980-985. |
| 37 | FAN Y, SIMMEN T. Mechanistic connections between endoplasmic reticulum (ER) redox control and mitochondrial metabolism[J/OL]. Cells, 2019, 8(9): 1071[2023-04-18]. . |
| 38 | CSORDÁS G, WEAVER D, HAJNÓCZKY G. Endoplasmic reticulum-mitochondrial contactology: structure and signaling functions[J]. Trends Cell Biol., 2018, 28(7): 523-540. |
| 39 | HETZ C, ZHANG K, KAUFMAN R J. Mechanisms, regulation and functions of the unfolded protein response[J]. Nat. Rev. Mol. Cell Biol., 2020, 21(8): 421-438. |
| 40 | PAN X, DALM C, WIJFFELS R H, et al.. Metabolic characterization of a CHO cell size increase phase in fed-batch cultures[J]. Appl. Microbiol. Biotechnol., 2017, 101(22): 8101-8113. |
| 41 | BUCHANAN A, CLEMENTEL V, WOODS R, et al.. Engineering a therapeutic IgG molecule to address cysteinylation, aggregation and enhance thermal stability and expression[J]. mAbs, 2013, 5(2): 255-262. |
| 42 | GARBER E, DEMAREST S J. A broad range of Fab stabilities within a host of therapeutic IgGs[J]. Biochem. Biophys. Res. Commun., 2007, 355(3): 751-757. |
| 43 | SHEN Y, ZENG L, ZHU A, et al.. Removal of a C-terminal serine residue proximal to the inter-chain disulfide bond of a human IgG1 lambda light chain mediates enhanced antibody stability and antibody dependent cell-mediated cytotoxicity[J]. mAbs, 2013, 5(3): 418-431. |
| 44 | KUANG B, DHARA V G, HOANG D, et al.. Identification of novel inhibitory metabolites and impact verification on growth and protein synthesis in mammalian cells[J/OL]. Metab. Eng. Commun., 2021, 13: e00182[2023-04-06]. . |
| 45 | REINHART D, DAMJANOVIC L, SOMMEREGGER W, et al.. Influence of cell culture media and feed supplements on cell metabolism and quality of IgG produced in CHO-K1, CHO-S, and CHO-DG44[J]. BMC Proc., 2015, 9(9): P36[2023-03-06]. . |
| 46 | EHRET J, ZIMMERMANN M, EICHHORN T, et al.. Impact of cell culture media additives on IgG glycosylation produced in Chinese Hamster Ovary cells[J]. Biotechnol. Bioeng., 2019, 116(4): 816-830. |
| 47 | HOSSLER P, RACICOT C, CHUMSAE C, et al.. Cell culture media supplementation of infrequently used sugars for the targeted shifting of protein glycosylation profiles[J]. Biotechnol. Prog., 2017, 33(2): 511-522. |
| 48 | LAKSHMANAN M, KOK Y J, LEE A P, et al.. Multi-omics profiling of CHO parental hosts reveals cell line-specific variations in bioprocessing traits[J]. Biotechnol. Bioeng., 2019, 116(9): 2117-2129. |
| 49 | 江一帆, 贾宇, 王龙, 等. 细胞培养过程对单克隆抗体糖基化修饰的影响和调控[J]. 中国生物工程杂志, 2019, 39(8): 95-103. |
| 50 | SHA S, AGARABI C, BRORSON K, et al.. N-glycosylation design and control of therapeutic monoclonal antibodies[J]. Trends Biotechnol., 2016, 34(10): 835-846. |
| 51 | BOUNE S, HU P, EPSTEIN A L, et al.. Principles of N-linked glycosylation variations of IgG-based therapeutics: pharmacokinetic and functional considerations[J/OL]. Antibodies, 2020, 9(2): 22[2022-02-18]. . |
| 52 | XU X, NAGARAJAN H, LEWIS N E, et al.. The genomic sequence of the Chinese Hamster Ovary (CHO)-K1 cell line[J]. Nat. Biotechnol., 2011, 29(8): 735-741. |
| 53 | LEE J S, KALLEHAUGE T B, PEDERSEN L E, et al.. Site-specific integration in CHO cells mediated by CRISPR/Cas9 and homology-directed DNA repair pathway[J/OL]. Sci. Rep., 2015, 5: 8572[2023-04-06]. . |
| 54 | 瞿丽丽, 丁学峰, 蔡燕飞, 等. CHO细胞基因组NW-003614092.1内稳定表达位点的发现[J]. 中国生物工程杂志, 2022, 42(6): 12-19. |
| 55 | O'BRIEN S A, OJHA J, WU P, et al.. Multiplexed clonality verification of cell lines for protein biologic production[J/OL]. Biotechnol. J., 2020, 36(4): e2978[2022-12-06]. . |
| 56 | CHUSAINOW J, YANG Y S, YEO J H M, et al.. A study of monoclonal antibody-producing CHO cell lines: what makes a stable high producer?[J]. Biotechnol. Bioeng., 2009, 102(4): 1182-1196. |
| 57 | ZEH N, SCHLOSSBAUER P, RAAB N, et al.. Cell line development for continuous high cell density biomanufacturing: exploiting hypoxia for improved productivity[J/OL]. Metab. Eng. Commun., 2021, 13: e00181[2023-03-06]. . |
| 58 | LIU L. Antibody glycosylation and its impact on the pharmacokinetics and pharmacodynamics of monoclonal antibodies and Fc-fusion proteins[J]. J. Pharm. Sci., 2015, 104(6): 1866-1884. |
| 59 | 王欢, 牛昆, 江一帆, 等. 重组单克隆抗体电荷异质性和工艺调控[J]. 生物技术进展, 2020, 10(5): 456-462. |
| [1] | Zhaohui CUI, Ling GUO, Xudong SHEN, Yi LIN, Lili ZHAI. Research Progress on Yeast Expression of Therapeutic Recombinant Proteins [J]. Current Biotechnology, 2025, 15(3): 380-387. |
| [2] | Xinran CHENG, Weiyong ZONG, Wenfang DOU. Research on the Synthesis of Cinnamyl Alcohol Glycosylated Derivatives Based on Immobilized Enzyme Method [J]. Current Biotechnology, 2025, 15(1): 110-118. |
| [3] | Li CAO, Shun LUO, Shihai XING, Jinshu QIU, Zhiyong LIN, Jun LIN, Xu MENG, Feng LIU. Effects of Dioscorea opposita Extract on CHO Cell Growth and Monoclonal Antibody Expression [J]. Current Biotechnology, 2023, 13(3): 449-456. |
| [4] | Zhekang JIA, Xinran CHENG, Wenfang DOU. Cascade Catalysis of Glycosyltransferase and Sucrose Synthase to Produce Astragalin [J]. Current Biotechnology, 2023, 13(2): 247-256. |
| [5] | Yiqi YANG, Zhigao ZHANG, Xiaolong YOU, Jing ZHANG, Guanfeng LIN, Yingsong WU. Development and Application of Monoclonal Antibody-based Drug [J]. Current Biotechnology, 2022, 12(3): 358-365. |
| [6] | PANG Liran, HE Cheng*, WEI Jingshuang, GAO Jian. Research Strategy on Long-acting Technology of Protein and Peptide Drugs [J]. Curr. Biotech., 2021, 11(3): 304-310. |
| [7] | ZHANG Bohui, JIA Daihui, CHENG Qian, XU Junyan*, SHAO Zhe, HUANG Yingfeng. Optimization of Hydrolysis Conditions of N-glycan from Monoclonal Antibodies by PNGase F [J]. Curr. Biotech., 2021, 11(2): 214-222. |
| [8] | DU Li, LIU Xiaozhi, WEI Jinshuang, GAO Jian*. Research Progress of Glycosylation Engineering of Protein Drug [J]. Curr. Biotech., 2020, 10(5): 448-455. |
| [9] | WANG Huan§, NIU Kun§, JIANG Yifan, DONG Jing*. Charge Heterogeneity and Process Control of Recombinant Monoclonal Antibodies [J]. Curr. Biotech., 2020, 10(5): 456-462. |
| [10] | HE Jinxia1,2, JIA Xiaochen2, WANG Wenxia2, HU Jianen1, YIN Heng2*. Progress on Detection Technology of N-glycan in Plant [J]. Curr. Biotech., 2018, 8(6): 500-508. |
| [11] | LV Ruo-yun§, WANG Hui§, CHEN Chen, ZHANG Shi-xiong, ZHANG Xiao-nan, CAO Chen-hua, WEI Jing-shuang*. Effect of the N-glycan Removal on the Neutralization Activity of Recombinant Anti-rabies Monoclonal Antibodies [J]. Curr. Biotech., 2016, 6(4): 277-282. |
| [12] | TAO Wei-hong, QIN Min-min, ZHANG Zhe-ru. Strategy Discussion for CHO Cell Line Development [J]. Curr. Biotech., 2014, 4(6): 394-399. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||