Current Biotechnology ›› 2024, Vol. 14 ›› Issue (4): 537-544.DOI: 10.19586/j.2095-2341.2024.0017
• Reviews • Previous Articles Next Articles
Enzymes Immobilization Technology and its Application Progress in Food Industry
Chen ZHONG1( ), Binxu ZHAO2, Mei LIU1, Tianhong LIU1, Ying WANG1,3(
), Binxu ZHAO2, Mei LIU1, Tianhong LIU1, Ying WANG1,3( )
)
- 1.Marine Science Research Institute of Shandong Province,Shandong Qingdao 266104,China
2.Qingdao University,Shandong Qingdao 266071,China
3.Municipal Engineering Research Center of Aquatic Biological Quality Evaluation and Application,Shandong Qingdao 266104,China
-
Received:2024-02-01Accepted:2024-03-27Online:2024-07-25Published:2024-08-07 -
Contact:Ying WANG
酶固定化技术及其在食品工业的应用进展
仲晨1( ), 赵彬旭2, 刘梅1, 刘天红1, 王颖1,3(
), 赵彬旭2, 刘梅1, 刘天红1, 王颖1,3( )
)
- 1.山东省海洋科学研究院,山东 青岛 266104
2.青岛大学,山东 青岛 266071
3.青岛市水产生物品质评价工程研究中心,山东 青岛 266104
-
通讯作者:王颖 -
作者简介:仲晨E-mail: 1369047180@qq.com; -
基金资助:山东省重点研发计划项目(2023CXGC010414);山东省重点研发计划-山东省“合成生物”科技示范工程(2022SFGC0101)
CLC Number:
Cite this article
Chen ZHONG, Binxu ZHAO, Mei LIU, Tianhong LIU, Ying WANG. Enzymes Immobilization Technology and its Application Progress in Food Industry[J]. Current Biotechnology, 2024, 14(4): 537-544.
仲晨, 赵彬旭, 刘梅, 刘天红, 王颖. 酶固定化技术及其在食品工业的应用进展[J]. 生物技术进展, 2024, 14(4): 537-544.
share this article
| 酶的名称 | 食品底物/产物 | 固定化方法 | 应用 |
|---|---|---|---|
| 菊粉酶 | 菊粉/果糖、低聚果糖 | 共价法、包封法 | 生产果糖糖浆、乙醇、丙酮和丁醇,用于降低糖尿病、龋齿和肥胖的风险[ |
| β-乳糖酶 | 乳糖/葡萄糖、半乳糖 | 吸附法 | 生产低乳糖和无乳糖产品[ |
| α-淀粉酶 | 玉米和马铃薯淀粉/低聚糖 | 包封法、共价/交联法 | 生产葡萄糖和果糖,用于糖果行业[ |
| 葡萄糖淀粉酶 | 淀粉/低聚糖 | 包封法 | 生产葡萄糖和果糖,用于糖果以及果汁工业[ |
| 脂肪酶 | 甘油三酯/甘油、脂肪酸 | 包封法 | 澄清果汁和葡萄酒;将植物油转化为人造黄油,生产奶酪和奶酪制品;在面包制作中减缓面包硬化[ |
| β-呋喃果糖苷酶 | 蔗糖/葡萄糖、果糖 | 包封法 | 防止果酱、糖果类产品在储存过程中糖化[ |
| L-苯丙氨酸解氨酶 | L-苯丙氨酸/抗坏血酸、氨 | 包封法 | 生产不含苯丙氨酸的产品[ |
| 天冬酰胺酶 | 丙烯酰胺/丙烯酸、氨 | 包封法 | 测定产品中丙烯酰胺含量[ |
| 脲酶 | 尿素/二氧化碳、氨 | 包封法 | 用于生物试验中控制牛奶质量[ |
Table 1 Summary of enzymes immobilization in food industry
| 酶的名称 | 食品底物/产物 | 固定化方法 | 应用 |
|---|---|---|---|
| 菊粉酶 | 菊粉/果糖、低聚果糖 | 共价法、包封法 | 生产果糖糖浆、乙醇、丙酮和丁醇,用于降低糖尿病、龋齿和肥胖的风险[ |
| β-乳糖酶 | 乳糖/葡萄糖、半乳糖 | 吸附法 | 生产低乳糖和无乳糖产品[ |
| α-淀粉酶 | 玉米和马铃薯淀粉/低聚糖 | 包封法、共价/交联法 | 生产葡萄糖和果糖,用于糖果行业[ |
| 葡萄糖淀粉酶 | 淀粉/低聚糖 | 包封法 | 生产葡萄糖和果糖,用于糖果以及果汁工业[ |
| 脂肪酶 | 甘油三酯/甘油、脂肪酸 | 包封法 | 澄清果汁和葡萄酒;将植物油转化为人造黄油,生产奶酪和奶酪制品;在面包制作中减缓面包硬化[ |
| β-呋喃果糖苷酶 | 蔗糖/葡萄糖、果糖 | 包封法 | 防止果酱、糖果类产品在储存过程中糖化[ |
| L-苯丙氨酸解氨酶 | L-苯丙氨酸/抗坏血酸、氨 | 包封法 | 生产不含苯丙氨酸的产品[ |
| 天冬酰胺酶 | 丙烯酰胺/丙烯酸、氨 | 包封法 | 测定产品中丙烯酰胺含量[ |
| 脲酶 | 尿素/二氧化碳、氨 | 包封法 | 用于生物试验中控制牛奶质量[ |
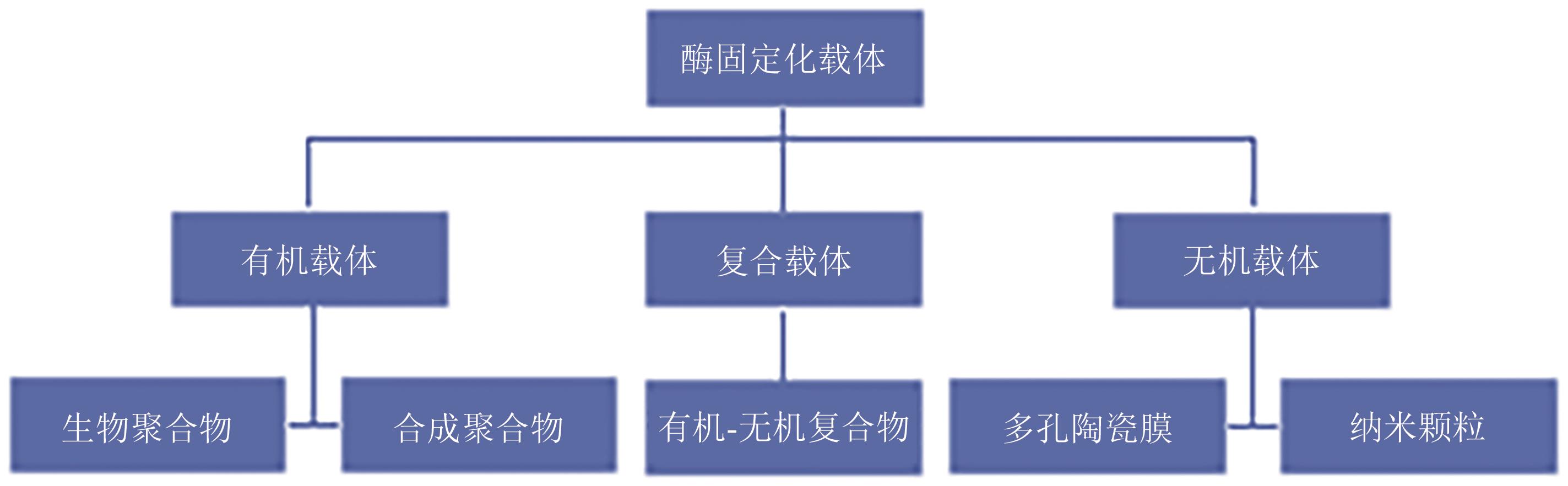
| 载体种类 | 载体材料 | 酶 | 初始酶活/% | 固定化后酶活 |
|---|---|---|---|---|
| 生物聚合物 | 海藻酸钙凝胶 | α-淀粉酶 | 80 | 75%(10次循环)[ |
| 海藻酸钙凝胶 | 葡萄糖氧化酶 | — | 37%(7次循环)[ | |
| 海藻酸钙凝胶 | 虫漆酶 | — | 70%(3次循环)[ | |
| 海藻酸钙凝胶 | 果胶酶 | 75 | 40%(6次循环)[ | |
| 明胶 | α-淀粉酶 | 40 | 46.8%(140 min)[ | |
| 壳聚糖 | β-半乳糖苷酶 | 85 | 70%(9次循环)[ | |
| 壳聚糖包被海藻酸盐 | 天冬酰胺酶 | — | 80%(4次循环)/40%(7次循环)[ | |
| 合成聚合物 | DEAE纤维素 | α-淀粉酶 | 68 | 49%(6次循环)[ |
| DEAE纤维素 | α-淀粉酶 | 84 | 96%(20次循环)[ | |
| 阴离子交换剂(DuoliteA568) | 菊粉酶 | 35.6 | 90%(3 h)[ | |
| 聚丙烯酰胺凝胶 | 菊粉酶 | 45 | 58%(96 h)[ | |
| PVA水凝胶 | 菊粉酶 | — | 60%(12次循环)/80%(3月)[ | |
| PVA水凝胶 | β-半乳糖苷酶 | — | 95%(7次循环)/51%(3月)[ | |
| PVA水凝胶 | 葡萄糖淀粉酶 | 35 | 80%(100次使用)[ | |
| 纳米颗粒 | Fe3O4纳米颗粒 | 脂肪酶 | — | 90%(10次循环)[ |
| Fe3O4纳米颗粒 | 脂肪酶 | — | 65%(7次循环)[ | |
| Fe3O4纳米颗粒 | 菊粉酶 | — | 70%(12次循环)[ | |
| SiO2纳米颗粒 | β-半乳糖苷酶 | 119 | 50%(6 h)/50%(9次循环)[ | |
| SiO2纳米颗粒 | β-半乳糖苷酶 | 120 | 71%(13次循环)[ | |
| Fe3O4-SiO2纳米颗粒 | α-淀粉酶 | — | 82%(10次循环)/74%(20次循环)[ | |
| 银纳米颗粒 | β-半乳糖苷酶 | 96 | 88%(6次循环)/83%(2月)[ | |
| 多孔陶瓷膜 | 氧化铝膜 | 脲酶 | — | 50%(5次循环)[ |
| 氧化铝膜 | 脂肪酶 | — | 65%(4次循环)[ | |
| 介孔SiO2 | β-半乳糖苷酶 | 40~65 | 50%(18 h)[ | |
| 复合物 | 壳聚糖包被AFSMNPs | α-淀粉酶 | — | 91%(10次循环)/85%(20次循环)[ |
| SiO2/壳聚糖复合物 | β-半乳糖苷酶 | 45~60 | 90%(200 h)[ |
Table 2 Effect of different carrier materials of enzyme immobilization in food industry
| 载体种类 | 载体材料 | 酶 | 初始酶活/% | 固定化后酶活 |
|---|---|---|---|---|
| 生物聚合物 | 海藻酸钙凝胶 | α-淀粉酶 | 80 | 75%(10次循环)[ |
| 海藻酸钙凝胶 | 葡萄糖氧化酶 | — | 37%(7次循环)[ | |
| 海藻酸钙凝胶 | 虫漆酶 | — | 70%(3次循环)[ | |
| 海藻酸钙凝胶 | 果胶酶 | 75 | 40%(6次循环)[ | |
| 明胶 | α-淀粉酶 | 40 | 46.8%(140 min)[ | |
| 壳聚糖 | β-半乳糖苷酶 | 85 | 70%(9次循环)[ | |
| 壳聚糖包被海藻酸盐 | 天冬酰胺酶 | — | 80%(4次循环)/40%(7次循环)[ | |
| 合成聚合物 | DEAE纤维素 | α-淀粉酶 | 68 | 49%(6次循环)[ |
| DEAE纤维素 | α-淀粉酶 | 84 | 96%(20次循环)[ | |
| 阴离子交换剂(DuoliteA568) | 菊粉酶 | 35.6 | 90%(3 h)[ | |
| 聚丙烯酰胺凝胶 | 菊粉酶 | 45 | 58%(96 h)[ | |
| PVA水凝胶 | 菊粉酶 | — | 60%(12次循环)/80%(3月)[ | |
| PVA水凝胶 | β-半乳糖苷酶 | — | 95%(7次循环)/51%(3月)[ | |
| PVA水凝胶 | 葡萄糖淀粉酶 | 35 | 80%(100次使用)[ | |
| 纳米颗粒 | Fe3O4纳米颗粒 | 脂肪酶 | — | 90%(10次循环)[ |
| Fe3O4纳米颗粒 | 脂肪酶 | — | 65%(7次循环)[ | |
| Fe3O4纳米颗粒 | 菊粉酶 | — | 70%(12次循环)[ | |
| SiO2纳米颗粒 | β-半乳糖苷酶 | 119 | 50%(6 h)/50%(9次循环)[ | |
| SiO2纳米颗粒 | β-半乳糖苷酶 | 120 | 71%(13次循环)[ | |
| Fe3O4-SiO2纳米颗粒 | α-淀粉酶 | — | 82%(10次循环)/74%(20次循环)[ | |
| 银纳米颗粒 | β-半乳糖苷酶 | 96 | 88%(6次循环)/83%(2月)[ | |
| 多孔陶瓷膜 | 氧化铝膜 | 脲酶 | — | 50%(5次循环)[ |
| 氧化铝膜 | 脂肪酶 | — | 65%(4次循环)[ | |
| 介孔SiO2 | β-半乳糖苷酶 | 40~65 | 50%(18 h)[ | |
| 复合物 | 壳聚糖包被AFSMNPs | α-淀粉酶 | — | 91%(10次循环)/85%(20次循环)[ |
| SiO2/壳聚糖复合物 | β-半乳糖苷酶 | 45~60 | 90%(200 h)[ |
| 1 | THOMPSON M, PEÑAFIEL I, COSGROVE S C, et al.. Biocatalysis using immobilized enzymes in continuous flow for the synthesis of fine chemicals[J]. Org. Proc. Res. Dev., 2019, 23(1): 9-18. |
| 2 | MALHOTRA M, KALLURI A, KUMAR C V. Nanoarmored multi-enzyme cascade catalysis[J]. Meth. Mol. Biol., 2022, 2487: 205-225. |
| 3 | SRIWONG K T, MATSUDA T. Recent advances in enzyme immobilization utilizing nanotechnology for biocatalysis[J]. Org. Proc. Res. Dev., 2022, 26(7): 1857-1877. |
| 4 | MADHAVAN A, SINDHU R, BINOD P, et al.. Strategies for design of improved biocatalysts for industrial applications[J]. Bioresour. Technol., 2017, 245(pt b): 1304-1313. |
| 5 | DICOSIMO R, MCAULIFFE J, POULOSE A J, et al.. Industrial use of immobilized enzymes[J]. Chem. Soc. Rev., 2013, 42(15): 6437-6474. |
| 6 | ZDARTA J, MEYER A S, JESIONOWSKI T, et al.. Developments in support materials for immobilization of oxidoreductases: a comprehensive review[J]. Adv. Colloid Interface Sci., 2018, 258: 1-20. |
| 7 | ZHAO F, WANG Q, DONG J, et al.. Enzyme-inorganic nanoflowers/alginate microbeads: an enzyme immobilization system and its potential application [J]. Proc. Biochem., 2017,57:87-94. |
| 8 | DATTA S, CHRISTENA L R, RAJARAM Y R S. Enzyme immobilization: an overview on techniques and support materials[J]. 3 Biotech, 2013, 3(1): 1-9. |
| 9 | NISHA S, KARTHICK S A, GOBI N. Application and properties of immobilized enzyme [J]. Chem. Sci. Rev. Lett., 2012, 1(3):148-155. |
| 10 | YUSHKOVA E, NAZAROVA E A, MATYUHINA A V, et al.. Application of immobilized enzymes in food industry[J]. J. Agric. Food Chem., 2019, 67(42): 11553-11567. |
| 11 | PRAMANIK S A N. Application of immobilized enzymes in the food industry [J]. Enzym. Food. Biotechnol., 2019, 42:711-721. |
| 12 | NESTORSON A, NEON K G, KANG E T, et al.. Enzyme immobilization in latex dispersion coatings for active food packaging [J]. Packag. Technol. Sci., 2008, 21(4):193-205. |
| 13 | RHIM J W, PARK H M, HA C S. Bio-nanocomposites for food packaging applications [J]. Prog. Polym. Sci., 2013,38(10-11):1629-1652. |
| 14 | MONTEREALI M R, DELIA SETA L, VASTARELLA W. A disposable laccase-tyrosinase based biosensor for amperometric detection of phenolic compounds in must and wine [J]. J. Mol. Catal. B-enzym., 2010, 64(3/4): 189-194. |
| 15 | YEWALE T, SINGHAL R S, VAIDYA A A.Immobilization of inulinase from Aspergillus niger NCIM 945 on chitosan and its application in continuous inulin hydrolysis [J]. Biocatal. Agric. Biotechnol., 2013, 2(2): 96-101. |
| 16 | CATANA R, FERREIRA B S, CABRAL J M S, et al.. Immobilization of inulinase for sucrose hydrolysis [J]. Food. Chem., 2005, 91(3): 517-520. |
| 17 | FISCHER C, KLEINSCHMIDT T. Synthesis of galactooligosaccharides in milk and whey: a review[J]. Comp. Rev. Food Sci. Food Saf., 2018, 17(3): 678-697. |
| 18 | GILLE D, WALTHER B, BADERTSCHER R, et al.. Detection of lactose in products with low lactose content [J]. Int. Dairy. J., 2018, 83: 17-19. |
| 19 | WON K, KIM S, KIM K J, et al.. Optimization of lipase entrapment in Ca-alginate gel beads [J]. Proc. Biochem., 2005, 40(6): 2149-2154. |
| 20 | CHIANG C J, HSIAU L T, LEE W C. Immobilization of cell-associated enzymes by entrapment in polymethacrylamide beads[J]. Biotechnol. Tech., 1997, 11(2): 121-125. |
| 21 | XU F, ORUNA-CONCHA M J, ELMORE J S. The use of asparaginase to reduce acrylamide levels in cooked food[J]. Food Chem., 2016, 210: 163-171. |
| 22 | SUJOY B, Enzymology APARNA A., immobilization and applications of urease enzyme [J]. Int. Res. J. Biol. Sci., 2013, 2(6): 2278-3202. |
| 23 | CABRERA M P, FONSECA T F, DE SOUZA R V B, et al.. Polyaniline-coated magnetic diatomite nanoparticles as a matrix for immobilizing enzymes[J]. Appl. Surf. Sci., 2018, 457: 21-29. |
| 24 | SPAHN C, MINTEER S. Enzyme immobilization in biotechnology [J]. Recent Pat. Eng., 2008, 2(3): 195-200. |
| 25 | MOHAMAD N R, MARZUKI N H, BUANG N A, et al.. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes[J]. Biotechnol. Biotechnol. Equip., 2015, 29(2): 205-220. |
| 26 | PRYAKHIN A N, CHUKHRAI E S. Glucose 6-phosphate dehydrogenase immobilized by adsorption on silica gel solid supports [J]. Vestn. Mosk. Univ., 1977, 18(1): 6. |
| 27 | SHEN Q, YANG R, HUA X, et al.. Gelatin-templated biomimetic calcification for β-galactosidase immobilization [J]. Proc. Biochem., 2011, 46(8): 1565-1571. |
| 28 | GORECKA E, JASTRZEBSKA M. Carriers-a review [J]. Biotechnol. Food. Sci., 2011, 75: 27-34. |
| 29 | KNEZEVIC Z, MILOSAVIC N, BEZBRADICA D, et al.. Immobilization of lipase from Candida rugosa on Eupergit C supports by covalent attachment [J]. Biochem. Eng. J., 2006, 30(3):269-278. |
| 30 | YANG Y, PORTE M C, MARMEY P, et al.. Covalent bonding of collagen on poly(L-lactic acid) by gamma irradiation [J]. Nucl. Instrum. Methods. Phys. Res. Sect. B., 2003, 207(2): 165-174. |
| 31 | SIGURDARDOTTIR S B, LEHMANN J, OVTAR S, et al.. Enzyme immobilization on inorganic surfaces for membrane reactor applications: mass transfer challenges, enzyme leakage and reuse of materials [J]. Adv. Synth. Catal., 2018, 360(14): 2578-2607. |
| 32 | OVSEJEVI K, MANTA C, BATISTA-VIERA F. Reversible covalent immobilization of enzymes via disulfide bonds[J]. Meth. Mol. Biol., 2013, 1051: 89-116. |
| 33 | COSTA S A, AZEVEDO H S, REIS R L. Enzyme immobilization in biodegradable polymers for biomedical applications [M]. Crc Press Taylor Francis Group, 2005: 957-979. |
| 34 | TEE B L, KALETUNÇ G. Immobilization of a thermostable alpha-amylase by covalent binding to an alginate matrix increases high temperature usability[J]. Biotechnol. Prog., 2009, 25(2): 436-445. |
| 35 | RUIZ E, BUSTO M D, RAMOS-GOMEZ S, et al.. Encapsulation of glucose oxidase in alginate hollow beads to reduce the fermentable sugars in simulated musts [J]. Food. Biosci., 2018, 24: 67-72. |
| 36 | OLAJUYIGBE F M, ADETUYI O Y, FATOKUN C O. Characterization of free and immobilized laccase from Cyberlindnera fabianii and application in degradation of bisphenol A[J]. Int. J. Biol. Macromol., 2019, 125: 856-864. |
| 37 | MARTIN M C, LOPEZ O V, CIOLINO A E, et al.. Immobilization of enological pectinase in calcium alginate hydrogels: a potential biocatalyst for winemaking [J]. Biocatal. Agric. Biotechnol., 2019, 18: 101091. |
| 38 | BAYRAMOGLU Z, AKBULUT U, SUNGUR S. Immobilization of alpha-amylase into photographic gelatin by chemical cross-linking[J]. Biomaterials, 1992, 13(10): 704-708. |
| 39 | BEDADE D K, SUTAR Y B, SINGHAL R S. Chitosan coated calcium alginate beads for covalent immobilization of acrylamidase: process parameters and removal of acrylamide from coffee[J]. Food Chem., 2019, 275: 95-104. |
| 40 | SHUKLA R J, SINGH S P. Structural and catalytic properties of immobilized α-amylase from Laceyella sacchari TSI-2[J]. Int. J. Biol. Macromol., 2016, 85: 208-216. |
| 41 | KIKANI B A, PANDEY S, SINGH S P. Immobilization of the α-amylase of Bacillus amyloliquifaciens TSWK1-1 for the improved biocatalytic properties and solvent tolerance[J]. Bioproc. Biosyst. Eng., 2013, 36(5): 567-577. |
| 42 | SINGH R S, DHALIWAL R, PURI M. Production of high fructose syrup from Asparagus inulin using immobilized exoinulinase from Kluyveromyces marxianus YS-1[J]. J. Ind. Microbiol. Biotechnol., 2007, 34(10): 649-655. |
| 43 | GUPTA A K, RATHORE P, KAUR N, et al.. Production, thermal stability and immobilisation of inulinase from Fusarium oxysporum [J]. J. Chem. Technol. Biotechnol., 1990, 47(3): 245-257. |
| 44 | ANES J, FERNANDES P. Towards the continuous production of fructose syrups from inulin using inulinase entrapped in PVA-based particles [J]. Biocatal. Agric. Biotechnol.. 2014, 3(4): 296-302. |
| 45 | JOVANOVIC-MALINOVSKA R, FERNANDES P, WINKELHAUSEN E, et al.. Galacto-oligosaccharides synthesis from lactose and whey by β-galactosidase immobilized in PVA[J]. Appl. Biochem. Biotechnol., 2012, 168(5): 1197-1211. |
| 46 | REBRO M, ROSENBERG M, MLICHOVA Z, et al.. A simple entrapment of glucoamylase into LentiKats as an efficient catalyst for maltodextrin hydrolysis [J]. Enzyme. Microb. Technol., 2006, 39(4): 800-804. |
| 47 | LYU J, LI Z, MEN J, et al.. Covalent immobilization of Bacillus subtilis lipase A on Fe3O4 nanoparticles by aldehyde tag: an ideal immobilization with minimal chemical modification [J]. Proc. Biochem., 2019, 81: 63-69. |
| 48 | WANG J, MENG G, TAO K, et al.. Immobilization of lipases on alkyl silane modified magnetic nanoparticles: effect of alkyl chain length on enzyme activity[J/OL]. PLoS One, 2012, 7(8): e43478[2024-04-15]. . |
| 49 | TORABIZADEH H, MAHMOUDI A. Inulin hydrolysis by inulinase immobilized covalently on magnetic nanoparticles prepared with wheat gluten hydrolysates[J]. Biotechnol. Rep. Amst., 2018, 17: 97-103. |
| 50 | GOEL A, SINHA R, KHARE S K. Immobilization of A. oryzae β-galactosidase on silica nanoparticles: development of an effective biosensor for determination of lactose in milk whey [J]. Recent Adv. Appl. Microbiol., 2017, 1: 3-18. |
| 51 | BENIWAL A, SAINI P, KOKKILIGADDA A, et al.. Use of silicon dioxide nanoparticles for β-galactosidase immobilization and modulated ethanol production by co-immobilized K. marxianus and S. cerevisiae in deproteinized cheese whey [J]. LWT-Food. Sci. Technol., 2018, 87: 553-561. |
| 52 | HOSSEINIPOUR S L, KHIABANI M S, HAMISHEHKAR H, et al.. Enhanced stability and catalytic activity of immobilized α-amylase on modified Fe3O4 nanoparticles for potential application in food industries[J/OL]. J. Nanopart. Res., 2015, 17(9): 382[2024-04-15]. . |
| 53 | ANSARI S A, SATAR R, ALAM F, et al.. Cost effective surface functionalization of silver nanoparticles for high yield immobilization of Aspergillus oryzae β-galactosidase and its application in lactose hydrolysis [J]. Proc. Biochem., 2012, 47(12): 2427-2433. |
| 54 | YANG Z, SI S, ZHANG C. Study on the activity and stability of urease immobilized onto nanoporous alumina membranes [J]. Microporous Mesoporous Mater., 2008, 111(1-3): 359-366. |
| 55 | MAGNAN E, CATARINO I, PAOLUCCI-JEANJEAN D, et al.. Immobilization of lipase on a ceramic membrane: activity and stability [J]. J. Membr. Sci., 2004, 241(1): 161-166. |
| 56 | BERNAL C, SIERRA L, MESA M. Improvement of thermal stability of β-galactosidase from Bacillus circulans by multipoint covalent immobilization in hierarchical macro-mesoporous silica [J]. J. Mol. Catal. B Enzym., 2012, 84: 166-172. |
| 57 | RICARDI N C, DE MENEZES E W, VALMIR BENVENUTTI E, et al.. Highly stable novel silica/chitosan support for β-galactosidase immobilization for application in dairy technology[J]. Food Chem., 2018, 246: 343-350. |
| 58 | TRAFFANO-SCHIFFO M V, CASTRO-GIRALDEZ M, et al.. Encapsulation of lactase in Ca(II)-alginate beads: Effect of stabilizers and drying methods [J]. Food. Res. Int., 2017, 100(Pt 1): 296-303. |
| 59 | SHVETSOVA S V, SHABALIN K A, BOBROV K S, et al.. Characterization of a new α-l-fucosidase isolated from Fusarium proliferatum LE1 that is regioselective to α-(1 → 4)-l-fucosidic linkage in the hydrolysis of α-l-fucobiosides[J]. Biochimie, 2017, 132: 54-65. |
| 60 | RASPOPOVA E A, KRASNOSHTANOVA A A. Characterizing the properties and evaluating the efficiency of biocatalysts based on immobilized fungal amylase[J]. Catal. Ind., 2016, 8(1): 75-80. |
| 61 | SIRISHA V L, JAIN A, JAIN A. Enzyme immobilization: an overview on methods, support material, and applications of immobilized enzymes[J]. Adv. Food Nutr. Res., 2016, 79: 179-211. |
| 62 | ROBINSON P J, DUNNILL P, LILLY M D. The properties of magnetic supports in relation to immobilized enzyme reactors [J]. Biotechnol. Bioeng., 1973, 15(3): 603-606. |
| 63 | VERMA M L, BARROW C J, PURI M. Nanobiotechnology as a novel paradigm for enzyme immobilisation and stabilisation with potential applications in biodiesel production[J]. Appl. Microbiol. Biotechnol., 2013, 97(1): 23-39. |
| 64 | LIU C, HONDA H, OHSHIMA A, et al.. Development of chitosan-magnetite aggregates containing Nitrosomonas europaea cells for nitrification enhancement[J]. J. Biosci. Bioeng., 2000, 89(5): 420-425. |
| 65 | CARLSSON N, GUSTAFSSON H, THÖRN C, et al.. Enzymes immobilized in mesoporous silica: a physical-chemical perspective[J]. Adv. Colloid Interface Sci., 2014, 205: 339-360. |
| 66 | HEILMANN A, TEUSCHER N, KIESOW A, et al.. Nanoporous aluminum oxide as a novel support material for enzyme biosensors[J]. J. Nanosci. Nanotechnol., 2003, 3(5): 375-379. |
| 67 | ASHTARI K, KHAJEH K, FASIHI J, et al.. Silica-encapsulated magnetic nanoparticles: enzyme immobilization and cytotoxic study[J]. Int. J. Biol. Macromol., 2012, 50(4): 1063-1069. |
| 68 | ARRUEBO M, FERNANDEZ-PACHECO R, IBARRA M R, et al. Magnetic nanoparticles for drug delivery [J]. Nano. Today, 2007, 2(3): 22-32. |
| 69 | FRAILE-GUTIÉRREZ I, IGLESIAS S, ACOSTA N, et al.. Chitosan-based oral hydrogel formulations of β-galactosidase to improve enzyme supplementation therapy for lactose intolerance[J/OL]. Int. J. Biol. Macromol., 2024, 255: 127755[2024-04-15]. . |
| 70 | ZHAO H, CUI Q, SHAH V, et al.. Enhancement of glucose isomerase activity by immobilizing on silica/chitosan hybrid microspheres [J]. J. Mol. Catal. B Enzym., 2016,126:18-23. |
| 71 | KAMANIN S S, ARLYAPOV V A, ROGOVA T V, et al.. Screen-printed electrodes modified with glucose oxidase immobilized in hybrid organosilicon sol-gel matrix[J]. Appl. Biochem. Microbiol., 2014, 50(9): 835-841. |
| 72 | REN S, LI C, JIAO X, et al.. Recent progress in multienzymes co-immobilization and multienzyme system applications [J]. Chem. Eng. J., 2019, 373: 1254-1278. |
| 73 | RODRIGUES R C, ORTIZ C, BERENGUER-MURCIA Á, et al.. Modifying enzyme activity and selectivity by immobilization[J]. Chem. Soc. Rev., 2013, 42(15): 6290-6307. |
| [1] | Xinran CHENG, Weiyong ZONG, Wenfang DOU. Research on the Synthesis of Cinnamyl Alcohol Glycosylated Derivatives Based on Immobilized Enzyme Method [J]. Current Biotechnology, 2025, 15(1): 110-118. |
| [2] | Xiaoni HOU, Mingdong LIU, Hao LYU, Deping YE, Lixia MA, Lihua ZHOU. Research Progress on Measuring Technology of Bio-enzyme Activity [J]. Current Biotechnology, 2025, 15(1): 58-66. |
| [3] | Xiaomin LIU, Ting LU, Yong LI, Meng WANG, Baokun ZHU, Wei ZHANG. The Impact of Enzyme Treatment on Yeast Fermentation of Tobacco [J]. Current Biotechnology, 2025, 15(1): 93-101. |
| [4] | Zhenyu WANG, Wenchao LU, Kangrong ZHONG, Yongjian GUAN, Zhen WANG, Chao CHEN. Preparation of Chondroitin Sulfate and Collagen Peptide of Sturgeon by Multi-enzyme Step-by-step Enzymatic Hydrolysis Under the Condition of pH Gradient [J]. Current Biotechnology, 2023, 13(6): 934-939. |
| [5] | Jie HAO, Xuanwen LI, Bao ZHANG, Chao ZHENG, Zhikang SUN, Qiang JI, Na WU, Han WU, Liqun LI. Application Progress of Cellulase in Tobacco [J]. Current Biotechnology, 2023, 13(2): 166-173. |
| [6] | Zhekang JIA, Xinran CHENG, Wenfang DOU. Cascade Catalysis of Glycosyltransferase and Sucrose Synthase to Produce Astragalin [J]. Current Biotechnology, 2023, 13(2): 247-256. |
| [7] | Ruiju MIAO, Zundan DING, Jian TIAN, Hongbing ZHANG, Feifei GUAN. Research Advances on Traditional and Intelligent Molecular Design of PET Hydrolases [J]. Current Biotechnology, 2023, 13(1): 46-54. |
| [8] | Yifei SONG, Fei XIE, Chen MA, Xuemei MA. Research Progress on Hydrogenase Activity in Higher Plants [J]. Current Biotechnology, 2022, 12(4): 481-489. |
| [9] | Haichao LYU, Zhekang JIA, Wen ZHANG, Liwen JIANG, Yuwen CHAO, Wenfang DOU. Study on Uridine Diphosphate Sugar Synthesis by Immobilized Enzyme [J]. Current Biotechnology, 2022, 12(2): 270-280. |
| [10] | Wenjing ZHANG, Ye TONG, Xiwen YANG, Yanjin CAO, Jidong WEI. Screening and Optimization of Highyield Neutral Proteaseproducing Strains and Pilot Scaleup [J]. Current Biotechnology, 2022, 12(1): 112-119. |
| [11] | Wanjie WANG, Nanzhu CHEN, Haisheng HAO, Xueming ZHAO, Huabin ZHU, Weihua DU. Research Progress of Histone Methyltransferases ASH2 [J]. Current Biotechnology, 2022, 12(1): 27-35. |
| [12] | Yage ZHANG, Yu PANG, Wei ZHANG, Zhengfu ZHOU. Global Patent Analysis of Lipase Development Trends [J]. Current Biotechnology, 2021, 11(6): 749-757. |
| [13] | Hui SUN, Chunyi ZHANG, Ling JIANG. Research Progress on Regulation of Coenzyme Ⅰ Metabolism [J]. Current Biotechnology, 2021, 11(4): 526-534. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||