Current Biotechnology ›› 2025, Vol. 15 ›› Issue (1): 1-10.DOI: 10.19586/j.2095-2341.2024.0121
• Reviews • Next Articles
Research Progress of Iron Signaling and its Role in Plant-pathogen Interaction
Wenxuan PU1( ), Xi DAI2, Jiani YUE3, Xiuxia FU4, Na SONG3, Wei LI3, Yu PENG1(
), Xi DAI2, Jiani YUE3, Xiuxia FU4, Na SONG3, Wei LI3, Yu PENG1( )
)
- 1.China Tobacco Hunan Industrial Co. ,Ltd. ,Changsha 410019,China
2.Hunan Tobacco Company Zhangjiajie Company,Hunan Zhangjiajie 427000,China
3.College of Plant Protection,Hunan Agricultural University,Changsha 410128,China
4.Tongjiang County Economic Crop Technology Promotion Station,Sichuan Bazhong 636700,China
-
Received:2024-07-02Accepted:2024-10-14Online:2025-01-25Published:2025-03-07 -
Contact:Yu PENG
铁信号及其在植物-病原物互作中的研究进展
蒲文宣1( ), 戴曦2, 岳佳妮3, 付秀霞4, 宋娜3, 李魏3, 彭宇1(
), 戴曦2, 岳佳妮3, 付秀霞4, 宋娜3, 李魏3, 彭宇1( )
)
- 1.湖南中烟工业有限责任公司,长沙 410019
2.湖南省烟草公司张家界市公司,湖南 张家界 427000
3.湖南农业大学植物保护学院,长沙 410128
4.四川省通江县经济作物技术推广站,四川 巴中 636700
-
通讯作者:彭宇 -
作者简介:蒲文宣E-mail: puwenxuan0313@163.com; -
基金资助:湖南中烟工业有限责任公司项目(KY2022YC0005);湖南省教育厅重点项目(21A0128);湖南省自然科学基金(2022JJ40178)
CLC Number:
Cite this article
Wenxuan PU, Xi DAI, Jiani YUE, Xiuxia FU, Na SONG, Wei LI, Yu PENG. Research Progress of Iron Signaling and its Role in Plant-pathogen Interaction[J]. Current Biotechnology, 2025, 15(1): 1-10.
蒲文宣, 戴曦, 岳佳妮, 付秀霞, 宋娜, 李魏, 彭宇. 铁信号及其在植物-病原物互作中的研究进展[J]. 生物技术进展, 2025, 15(1): 1-10.
share this article
| 1 | KOBAYASHI T, NOZOYE T, NISHIZAWA N K. Iron transport and its regulation in plants[J]. Free Radic. Bio. Med., 2019, 133: 11-20. |
| 2 | YONEYAMA T. Iron delivery to the growing leaves associated with leaf chlorosis in mugineic acid family phytosiderophores-generating graminaceous crops[J]. Soil Sci. Plant Nutr., 2021, (15): 1-12. |
| 3 | IMRAN M, SUN X, HUSSAIN S, et al.. Molybdenum-induced effects on nitrogen metabolism enzymes and elemental profile of winter wheat (Triticum aestivum L.) under different nitrogen sources[J/OL]. Int. J. Mol. Sci., 2019, 20(12): E3009[2025-1-10]. . |
| 4 | VOSE P B. Iron nutrition in plants: a world overview[J]. J. Plant Nut., 1982, 5(4-7): 233-249. |
| 5 | AUNG M S, MASUDA H. How does rice defend against excess iron? Physiological and molecular mechanisms[J/OL]. Front. Plant Sci., 2020, 11: 1102[2024-12-30]. . |
| 6 | ELBASUNEY S, EL-SAYYAD G S, ATTIA M S, et al.. Ferric oxide colloid:towards green nano-fertilizer for tomato plant with enhanced vegetative growth and immune response against Fusarium wilt disease[J]. J.Inorg.Organomet.Polym.Mater., 2022, 32(11): 4270-4283. |
| 7 | GUERINOT M L. It's elementary: enhancing Fe3+ reduction improves rice yields[J]. Proc. Natl. Acad. Sci. USA, 2007, 104(18):7311-7312. |
| 8 | TSAI H H, RODRÍGUEZ-CELMA J, LAN P, et al.. Scopoletin 8-hydroxylase-mediated fraxetin production is crucial for iron mobilization[J]. Plant Physiol., 2018, 177(1): 194-207. |
| 9 | ROBE K, CONEJERO G, GAO F, et al.. Coumarin accumulation and trafficking in Arabidopsis thaliana: a complex and dynamic process[J]. New Phytol., 2021, 229(4): 2062-2079. |
| 10 | ROBE K, IZQUIERDO E, VIGNOLS F, et al.. The coumarins:secondary metabolites playing a primary role in plant nutrition and health[J]. Trends Plant Sci., 2021, 26(3): 248-259. |
| 11 | HARBORT C J, HASHIMOTO M, INOUE H, et al.. Root-secreted coumarins and the microbiota interact to improve iron nutrition in Arabidopsis [J]. Cell Host Microbe, 2020, 28(6): 825-837. |
| 12 | DJAVAHERI M, MERCADO-BLANCO J, VERSLUIS C, et al.. Iron-regulated metabolites produced by Pseudomonas fluorescens WCS374r are not required for eliciting induced systemic resistance against Pseudomonas syringae pv.tomato in Arabidopsis [J]. MicrobiologyOpen, 2012, 1(3): 311-325. |
| 13 | LI L H, CHENG X D, LING H Q. Isolation and characterization of Fe(Ⅲ)-chelate reductase gene LeFRO1 in tomato[J]. Plant Mol. Biol., 2004, 54(1): 125-136. |
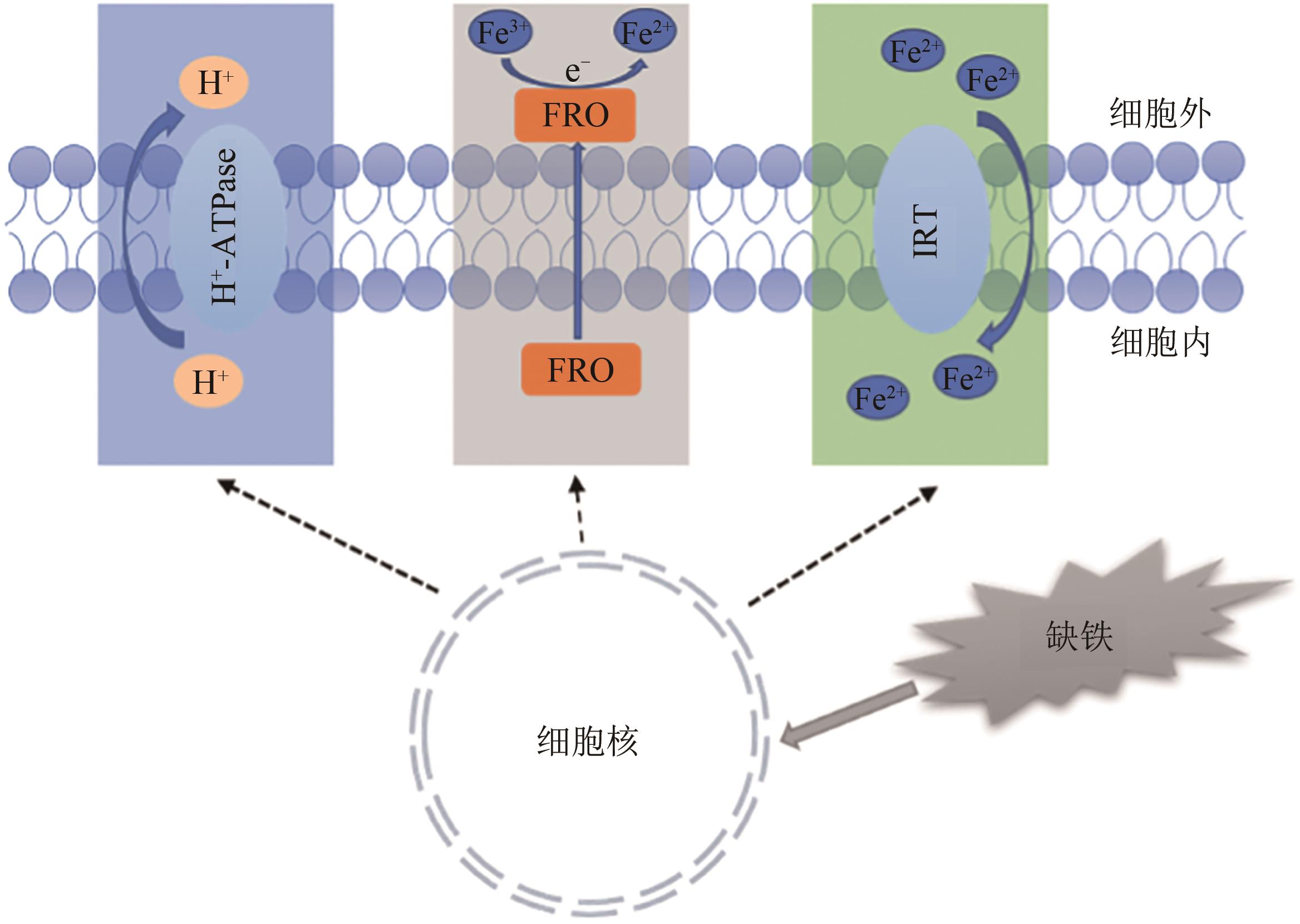
| 14 | WATERS B M, LUCENA C, ROMERA F J, et al.. Ethylene involvement in the regulation of the H(+)-ATPase CsHA1 gene and of the new isolated ferric reductase CsFRO1 and iron transporter CsIRT1 genes in cucumber plants[J]. Plant Physiol.Biochem., 2007, 45(5): 293-301. |
| 15 | FOURCROY P, TISSOT N, GAYMARD F, et al.. Facilitated Fe nutrition by phenolic compounds excreted by the Arabidopsis ABCG37/PDR9 transporter requires the IRT1/FRO2 high-affinity root Fe(2+) transport system[J]. Mol.Plant, 2016, 9(3):485-488. |
| 16 | DEY S, REGON P, KAR S, et al.. Chelators of iron and their role in plant's iron management[J]. Physiol.Mol.Biol.Plants, 2020, 26(8): 1541-1549. |
| 17 | HERLIHY J H, LONG T A, MCDOWELL J M. Iron homeostasis and plant immune responses:recent insights and translational implications[J]. J. Biol. Chem., 2020, 295(39):13444-13457. |
| 18 | WANG M, GONG J, BHULLAR N K. Iron deficiency triggered transcriptome changes in bread wheat[J]. Comput.Struct.Biotechnol. J., 2020, 18: 2709-2722. |
| 19 | DU X Y, WANG H N, HE J F, et al.. Identification of nicotianamine synthase genes in Triticum monococcum and their expression under different Fe and Zn concentrations[J]. Gene, 2018, 672: 1-7. |
| 20 | 李洪有,钟长春,蔡芳,等.甜荞柠檬酸转运蛋白基因FeFRD3的克隆及表达分析[J]. 西北植物学报,2018,38(3):409-415. |
| LI H Y, ZHONG C C, CAI F, et al.. Cloning and expression analysis of FeFRD3 citrate efflux transporter gene in Fagopyrum esculentum [J]. Acta Bot.Boreali. Occidentalia Sin., 2018, 38(3): 409-415. | |
| 21 | ZHU C Q, ZHANG J H, ZHU L F, et al.. NH4+ facilitates iron reutilization in the cell walls of rice (Oryza sativa) roots under iron-deficiency conditions[J]. Environ. Exp. Bot., 2018, 151: 21-31. |
| 22 | EIDE D, BRODERIUS M, FETT J, et al.. A novel iron-regulated metal transporter from plants identified by functional expression in yeast[J]. Proc.Natl.Acad.Sci.USA, 1996, 93(11):5624-5628. |
| 23 | CONTE S S, WALKER E L. Transporters contributing to iron trafficking in plants[J]. Mol. Plant, 2011, 4(3): 464-476. |
| 24 | LIN Y F, LIANG H M, YANG S Y, et al.. Arabidopsis IRT3 is a zinc-regulated and plasma membrane localized zinc/iron transporter[J]. New Phytol, 2009, 182(2): 392-404. |
| 25 | LI S, ZHOU X, LI H,et al.. Overexpression of ZmIRT1 and ZmZIP3 enhances iron and zinc accumulation in transgenic Arabidopsis [J/OL]. PLoS ONE, 2015, 10(8): e0136647[2024-12-30]. . |
| 26 | ECKHARDT U, MARQUES AMAS, BUCKHOUT T J. Two iron-regulated cation transporters from tomato complement metal uptake-deficient yeast mutants[J]. Plant Mol. Biol., 2001, 45(4): 437-448. |
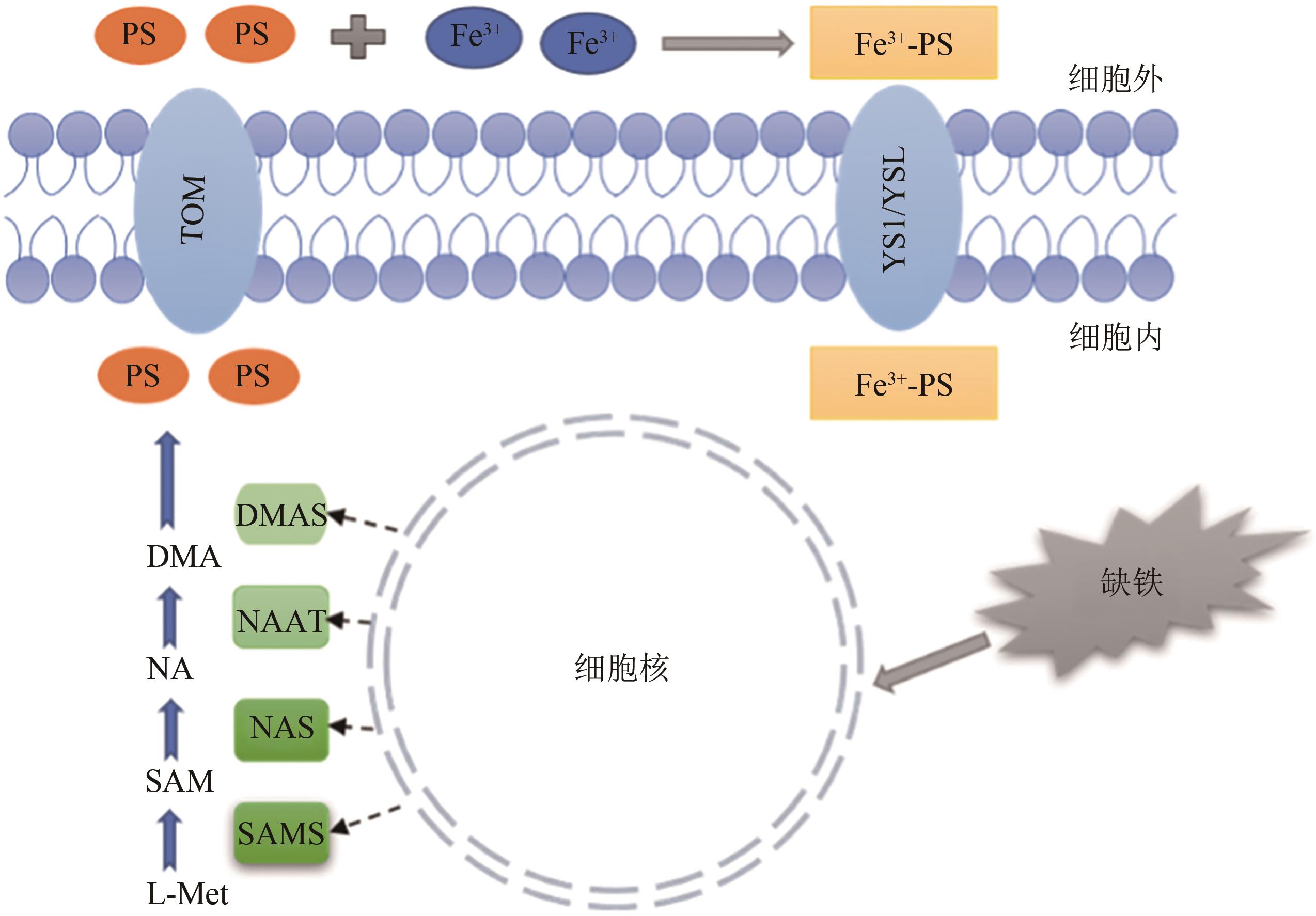
| 27 | KLATTE M, SCHULER M, WIRTZ M, et al.. The analysis of Arabidopsis nicotianamine synthase mutants reveals functions for nicotianamine in seed iron loading and iron deficiency responses[J]. Plant Physiol., 2009, 150(1): 257-271. |
| 28 | SCHULER M, RELLÁN-ÁLVAREZ R, FINK-STRAUBE C, et al.. Nicotianamine functions in the phloem-based transport of iron to sink organs,in pollen development and pollen tube growth in Arabidopsis [J]. Plant Cell, 2012, 24(6): 2380-2400. |
| 29 | INOUE H, HIGUCHI K, TAKAHASHI M, et al.. Three rice nicotianamine synthase genes,OsNAS1,OsNAS2,and OsNAS3 are expressed in cells involved in long-distance transport of iron and differentially regulated by iron[J]. Plant J., 2003, 36(3):366-381. |
| 30 | ZHOU M L, QI L P, PANG J F, et al.. Nicotianamine synthase gene family as central components in heavy metal and phytohormone response in maize[J]. Funct. Integr. Genom., 2013, 13(2):229-239. |
| 31 | REGON P, DEY S, CHOWARDHARA B, et al.. Physio-biochemical and molecular assessment of iron (Fe2+) toxicity responses in contrasting indigenous aromatic Joha rice cultivars of Assam, India[J]. Protoplasma, 2021, 258(2): 289-299. |
| 32 | KAKEI Y, ISHIMARU Y, KOBAYASHI T, et al.. OsYSL16 plays a role in the allocation of iron[J]. Plant Mol. Biol., 2012, 79(6): 583-594. |
| 33 | BASHIR K, NOZOYE T, NAGASAKA S, et al.. Paralogs and mutants show that one DMA synthase functions in iron homeostasis in rice[J]. J. Exp. Bot., 2017, 68(7): 1785-1795. |
| 34 | SENOURA T, SAKASHITA E, KOBAYASHI T, et al.. The iron-chelate transporter OsYSL9 plays a role in iron distribution in developing rice grains[J]. Plant Mol. Biol., 2017, 95(4):375-387. |
| 35 | CHU H H, CHIECKO J, PUNSHON T, et al.. Successful reproduction requires the function of Arabidopsis Yellow Stripe-Like1 and Yellow Stripe-Like3 metal-nicotianamine transporters in both vegetative and reproductive structures[J]. Plant Physiol., 2010, 154(1): 197-210. |
| 36 | JIA B, GUO G L, Yu T, et al.. Analysis of endogenous IAA content and siganling genes expression in retrieved leaves of ‘dangshansuli’ pear (Pyrus Bretschneideri Rehd)[J]. Acta Bot. Boreali-Occidentalia Sin., 2021, 041(004): 595-605. |
| 37 | LV X M, ZHANG Y X, HU L, et al.. Low-nitrogen stress stimulates lateral root initiation and nitrogen assimilation in wheat:roles of phytohormone signaling[J]. J.Plant Growth Regul., 2021, 40(1): 436-450. |
| 38 | ANGULO M, GARCÍA M J, ALCÁNTARA E, et al.. Comparative study of several Fe deficiency responses in the Arabidopsis thaliana ethylene insensitive mutants ein2-1 and ein2-5 [J]. Plants, 2021, 10(2): 262[2024-12-30]. . |
| 39 | ZANG J, HUO Y, LIU J, et al.. Maize YSL2 is required for iron distribution and development in kernels[J]. J.Exp.Bot., 2020, 71(19): 5896-5910. |
| 40 | FAN W, WANG H, WU Y, et al.. H+-pyrophosphatase IbVP1 promotes efficient iron use in sweet potato [Ipomoea batatas (L.) Lam.][J]. Plant Biotechnol. J., 2017, 15(6): 698-712. |
| 41 | 李鑫, 吴元华, 顾晶晶 等.铁诱导烟草抗PVYN的调控作用研究[J]. 沈阳农业大学学报, 2009, 40(1): 11-15. |
| LI X, WU Y H, GU J J, et al.. The resistant to PVYN and defensive enzymes of tobacco induced by iron[J]. J. Shenyang Agric. Univ., 2009, 40(1): 11-15. | |
| 42 | 李晔,吴元华,赵秀香,等.铁营养抑制烟草感染TMV及其对钙信使系统调控作用研究[J]. 植物营养与肥料学报,2007,13(5):920-924. |
| LI Y, WU Y H, ZHAO X X, et al.. Studies on the regulation of the inhibition of TMV infection and calcium signaling system with iron nutrient treatment[J]. J. Plant Nutr. Fertil., 2007, 13(5):920-924. | |
| 43 | MEENA M, SWAPNIL P, DIVYANSHU K, et al.. PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens:current perspectives[J]. J. Basic Microbiol., 2020, 60(10): 828-861. |
| 44 | DJAVAHERI M, MERCADO-BLANCO J, VERSLUIS C, et al.. Iron-regulated metabolites produced by Pseudomonas fluorescens WCS374r are not required for eliciting induced systemic resistance against Pseudomonas syringae pv.tomato in Arabidopsis [J]. MicrobiologyOpen, 2012, 1(3): 311-325. |
| 45 | PRESS C M, WILSON M, TUZUN S, et al.. Salicylic acid produced by Serratia marcescens 90-166 is not the primary determinant of induced systemic resistance in cucumber or tobacco[J]. Mol. Plant Microbe In., 2007, 10(6): 761-768. |
| 46 | DELLAGI A, SEGOND D, RIGAULT M, et al.. Microbial siderophores exert a subtle role in arabidopsis during infection by manipulating the immune response and the iron status[J]. Plant Physiol., 2009, 150(4): 1687-1696. |
| 47 | DIXON R A, ACHNINE L, KOTA P, et al.. The phenylpropanoid pathway and plant defence-a genomics perspective[J]. Mol. Plant Pathol., 2002, 3(5): 371-390. |
| 48 | XING Y, XU N, BJANDARI D D, et al.. Bacterial effector targeting of a plant iron sensor facilitates iron acquisition and pathogen colonization[J]. Plant Cell, 2021, 33(6): 2015-2031. |
| 49 | KIEU NP, AZNAR A, SEGOND D, et al.. Iron deficiency affects plant defence responses and confers resistance to Dickeya dadantii and Botrytis cinerea [J]. Mol. Plant Pathol., 2012, 13(8): 816-827. |
| 50 | HSIAO P Y, CHENG C P, KOH K W, et al.. The Arabidopsis defensin gene, AtPDF1.1, mediates defence against Pectobacterium carotovorum subsp. carotovorum via an iron-withholding defence system[J/OL]. Sci. Rep., 2017, 7(1): 9175[2024-11-30]. . |
| 51 | DELLAGI A, RIGAULT M, SEGOND D, et al.. Siderophore- mediated upregulation of Arabidopsis ferritin expression in response to Erwinia chrysanthemi infection[J]. Plant J., 2005, 43(2):262-272. |
| 52 | LU C K, LIANG G. Fe deficiency-induced ethylene synthesis confers resistance to Botrytis cinerea [J]. New Phytol., 2023, 237(5): 1843-1855. |
| 53 | PERKOWSKA I, POTRYKUS M, SIWINSKA J, et al.. Interplay between coumarin accumulation, iron deficiency and plant resistance to Dickeya spp.[J/OL]. Int. J. Mol. Sci., 2021, 22(12): 6449[2025-1-10]. . |
| 54 | LIU G, GREENSHIELDS D L, SAMMYNAIKEN R, et al.. Targeted alterations in iron homeostasis underlie plant defense responses[J]. J. Cell Sci., 2007, 120(Pt 4): 596-605. |
| 55 | WENNMAN A, OLIW E H, KARKEHABADI S, et al.. Crystal structure of manganese lipoxygenase of the rice blast fungus Magnaporthe oryzae [J]. J. Biol. Chem., 2016, 291(15): 8130-8139. |
| 56 | SÁNCHEZ-SANUY F, PERIS-PERIS C, TOMIYAMA S, et al.. Osa-miR7695 enhances transcriptional priming in defense responses against the rice blast fungus[J/OL]. BMC Plant Biol., 2019, 19(1): 563[2024-12-30]. . |
| 57 | CARROLL C S, MOORE M M. Ironing out siderophore biosyn-thesis: a review of non-ribosomal peptide synthetase (nrps)-independent siderophore synthetases[J]. Crit. Rev. Biochem. Mol. Biol., 2018, 53: 356-381. |
| 58 | OIDE S, MOEDER W, KRASNOFF S, et al.. NPS6, encoding a nonribosomal peptide synthetase involved in siderophore-mediated iron metabolism, is a conserved virulence determinant of plant pathogenic ascomycetes[J]. Plant Cell, 2006, 18(10): 2836-2853. |
| 59 | ELÍAS, JULIANA M, RAMÍREZ-MATA, et al.. The polar flagellin of Azospirillum brasilense REC3 induces a defense response in strawberry plants against the fungus Macrophomina phaseolina [J]. J. Plant Growth Regul., 2021, 41: 2992-3008. |
| 60 | EICHHORN H, LESSING F, WINTERBERG B, et al.. A ferroxidation/permeation iron uptake system is required for virulence in Ustilago maydis [J]. Plant Cell, 2006, 18(11): 3332-3345. |
| 61 | WANG L, PAN Y, YUAN Z H, et al.. Iron-VgrR binding disassociates VgrR-DNA and VgrR-VgrS interactions[J/OL]. PLoS Pathogens, 2016, 12[2024-12-10]. . |
| 62 | PAN Y, LIANG F, LI R J, et al.. MarR-family transcription factor HpaR controls expression of the vgrR-vgrS operon of Xanthomonas campestris pv. Campestris [J]. Mol. Plant Microbe Interact., 2018, 31(3): 299-310. |
| 63 | FRANZA T, EXPERT D. Role of iron homeostasis in the virulence of phytopathogenic bacteria: an ‘à la carte’ menu[J]. Mol. Plant Pathol., 2013, 14(4): 429-438. |
| 64 | KUZNETS G, VIGONSKY E, WEISSMAN Z, et al.. A relay network of extracellular heme-binding proteins drives C. albicans iron acquisition from hemoglobin[J/OL]. PLoS Pathog., 2014, 10(10): e1004407[2024-12-30]. . |
| 65 | ALBAROUKI E, DEISING H B. Infection structure-specific reductive iron assimilation is required for cell wall integrity and full virulence of the maize pathogen Colletotrichum graminicola [J]. Mol. Plant Microbe In., 2013, 26(6): 695-708. |
| 66 | EICHHORN H, LESSING F, WINTERBERG B, et al.. A ferroxidation/permeation iron uptake system is required for virulence in Ustilago maydis [J]. Plant Cell, 2006, 18(11): 3332-3345. |
| 67 | SHIRAZI F, KONTOYIANNIS D P, IBRAHIM A S. Iron starvation induces apoptosis in rhizopus oryzae in vitro[J]. Virulence, 2015, 6(2): 121-126. |
| 68 | LI Y Y, SUI X Y, YANG J S, et al.. A novel bHLH transcription factor, NtbHLH1, modulates iron homeostasis in tobacco (Nicotiana tabacum L.)[J]. Biochem. Biophys. Res. Commun., 2020, 522(1): 233-239. |
| 69 | YAO Z, HAO W, WANG Y, et al.. Loss-of-function mutations in the ERF96 gene enhance iron-deficient tolerance in Arabidopsis [J]. Plant Physiol. Biochem., 2022, 175: 1-11. |
| 70 | CAMPO S, PERIS-PERIS C, SIRÉ C, et al.. Identification of a novel microRNA (miRNA) from rice that targets an alternatively spliced transcript of the Nramp6 (Natural resistance-associated macrophage protein 6) gene involved in pathogen resistance[J]. New Phytol., 2013, 199(1): 212-227. |
| 71 | YE F, ALBAROUKI E, LINGAM B, et al.. An adequate Fe nutritional status of maize suppresses infection and biotrophic growth of Colletotrichum graminicola [J]. Physiol. Plantarum., 2014, 151 (3): 280-292. |
| 72 | DANGOL S, CHEN Y, HWANG B K, et al.. Iron-and reactive oxygen species-dependent ferroptotic cell death in rice-Magnaporthe oryzae interactions[J]. Plant Cell, 2019, 31(1): 189-209. |
| 73 | AZNAR A, PATRIT O, BERGER A, et al.. Alterations of iron distribution in Arabidopsis tissues infected by Dickeya dadantii [J]. Mol. Plant Pathol., 2014, 16(5): 521-528. |
| [1] | Xiaoyi ZHAI, Haiyue ZHANG, Wenjia GUO, Xiaogang DONG. Research Progress of Cancer-associated Fibroblasts in Breast Cancer [J]. Current Biotechnology, 2025, 15(4): 636-644. |
| [2] | Chuancai LIANG, Bo QIU. Echinoside Inhibits IL-1β-induced Chondrocytes Iron Death Through Nrf2/HO-1 Pathway [J]. Current Biotechnology, 2025, 15(4): 720-725. |
| [3] | Xiaoya LIU, Shuomin ZHANG, Peng ZHENG, Rui MA, Chaojun ZHANG. Role and Mechanisms of DBNDD1 in Colorectal Cancer Development [J]. Current Biotechnology, 2025, 15(4): 726-734. |
| [4] | Yanjie WANG, Bo QIU. The Role and Research Progress of Gene P53 in Osteosarcoma [J]. Current Biotechnology, 2025, 15(2): 241-246. |
| [5] | Shangshang WANG, Zhenya XUE, Haiyan XING, Xue YANG, Min WANG, Qing RAO. Influence of Different Lodging Sites on the Stemness of Leukemia Cells in a Mouse Leukemia Model [J]. Current Biotechnology, 2025, 15(2): 349-354. |
| [6] | Yeerkenbieke BUERLAN, Wenjia GUO, Xiaogang DONG. Advances on the Function of POSTN in Tumor Microenvironment [J]. Current Biotechnology, 2024, 14(2): 205-210. |
| [7] | Pengxiao ZHANG, Nian HU. The Research Progress on Action Mechanism of Melanoma Immunotherapy [J]. Current Biotechnology, 2023, 13(6): 900-906. |
| [8] | Wenbo MA, Yiqun PAN, Qun WANG, Zhuang MA, Minglian WANG, Yishu YANG. Preliminary Study on the Application of Graphene-coated Iron Nitride Magnetic Beads to Capture Lung Cancer Circulating Tumor Cells [J]. Current Biotechnology, 2023, 13(4): 628-636. |
| [9] | Yongchao LI, Zhao YANG. Status and Countermeasures of Bispecific Antibody Drugs [J]. Current Biotechnology, 2023, 13(3): 353-358. |
| [10] | Jiayi LIN, Ju XIE, Yueping ZHU, Wenyu XIE. Metabolomics and its Application in Environmental Toxicology [J]. Current Biotechnology, 2022, 12(5): 683-689. |
| [11] | Peimin LIU, Jinping LUO, Quanxin GAO. Research Progress of Environmental Microorganisms in Aquaculture [J]. Current Biotechnology, 2022, 12(5): 690-695. |
| [12] | Yichao LIU, Chao LU, Yuhua ZHAN, Xiubin KE, Wei LU, Yongliang YAN. Expressional and Functional Characterization of the Ferric Uptake Regulator Fur in Pseudomonas stutzeri A1501 [J]. Current Biotechnology, 2022, 12(3): 387-395. |
| [13] | Xin WU, Dan WAN, Yulong YIN. Research Progress on Calcium, Phosphorus and Trace Elements Nutrition in Livestock and Poultry [J]. Current Biotechnology, 2021, 11(4): 455-461. |
| [14] | LIU Shibo1, WU Hao1, HONG Jiao2, LIU Mengyu1, YAO Mawulikplimi Adzavon1, ZHAO Pengxiang1*. Advances in the Study of Inflammation-tumor Transformation in Ocular Diseases [J]. Curr. Biotech., 2020, 10(3): 234-241. |
| [15] | ZHANG Zaibao1,2§, ZHAO Hai1§, HU Menghui1, DENG Lijun1, WANG Qi1, LI Jiuli1, YUAN Hongyu1,2. Application Progress of Omics in the Research of Anther Development Ⅰ: Transcriptomics [J]. Curr. Biotech., 2019, 9(5): 433-439. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||