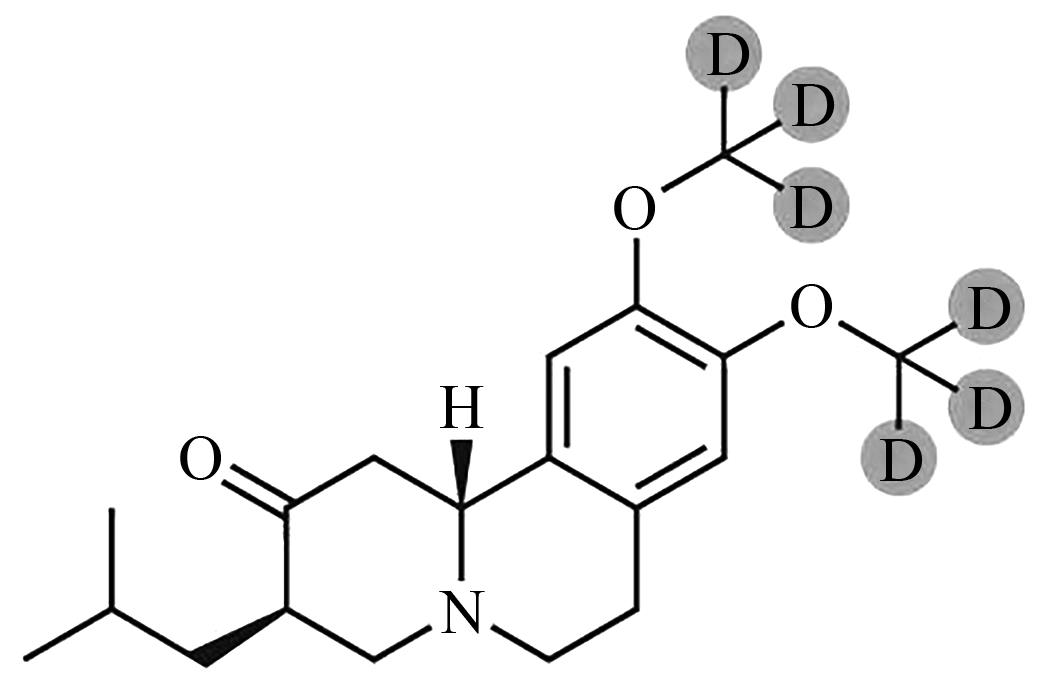
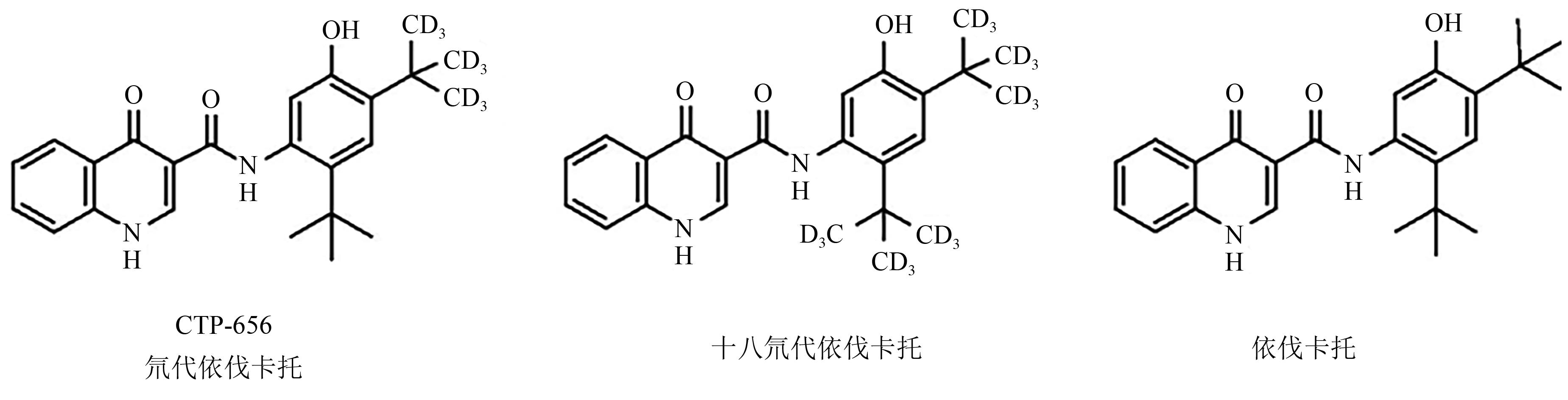
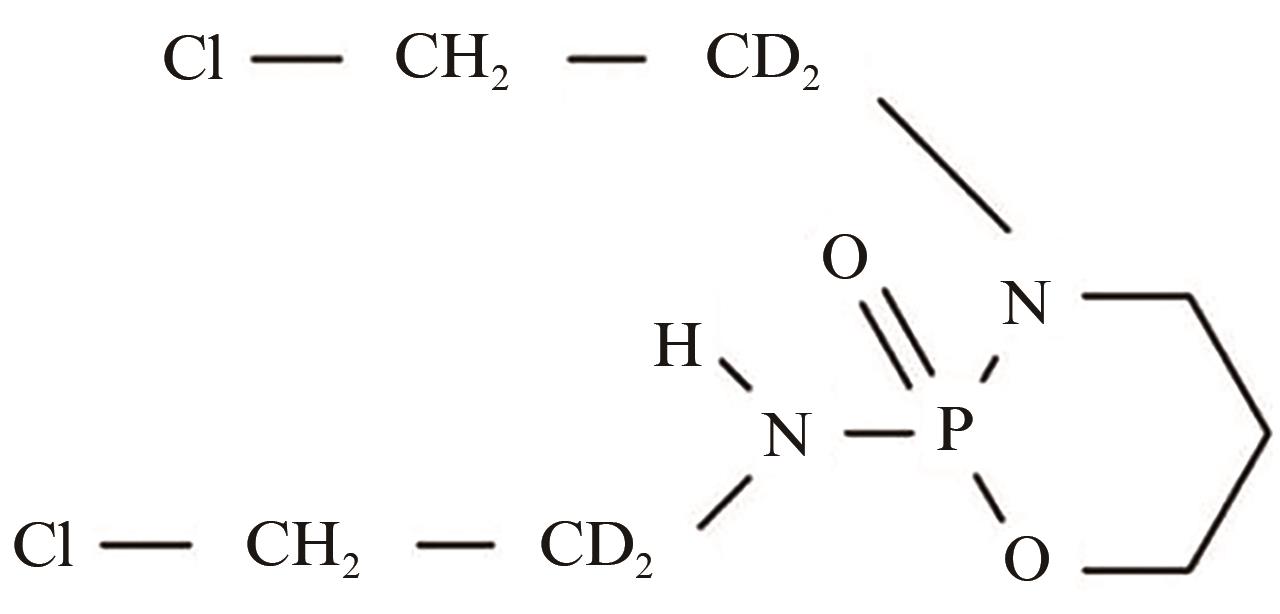
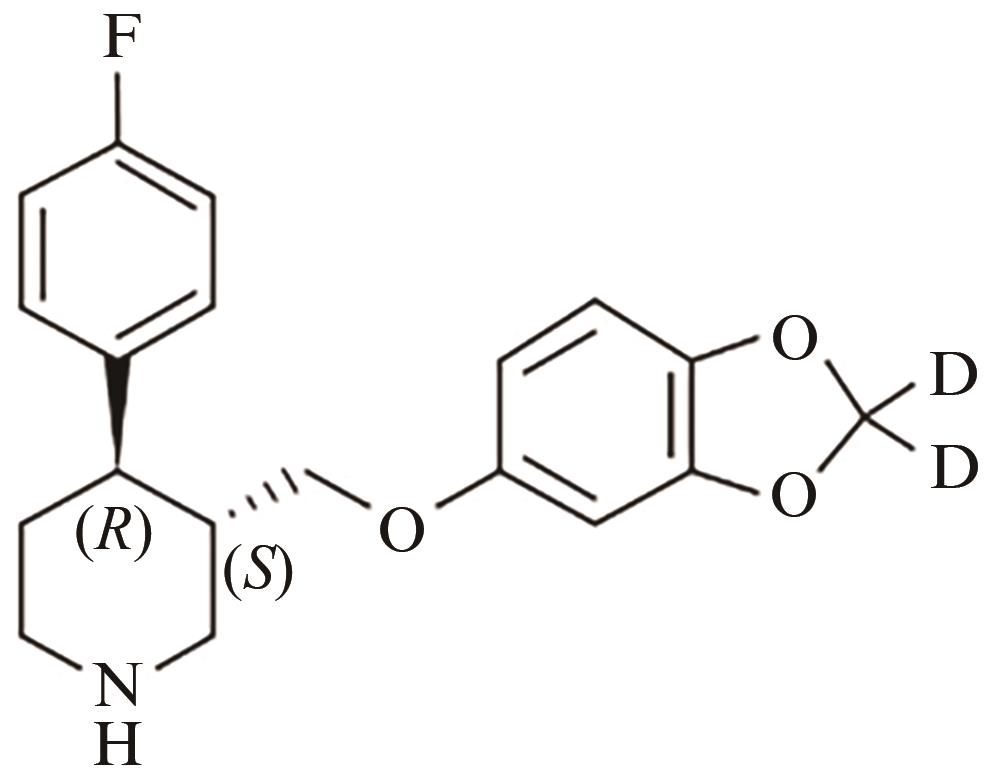
Current Biotechnology ›› 2024, Vol. 14 ›› Issue (5): 785-792.DOI: 10.19586/j.2095-2341.2024.0023
• Reviews • Previous Articles Next Articles
Progress in the Application of Deuterium and its Labeled Compounds in Biomedical Research
Jinge TANG1,2( ), Wei REN2, Qingqing JIANG2, Ning YU1(
), Wei REN2, Qingqing JIANG2, Ning YU1( )
)
- 1.North Sichuan Medical College,Sichuang Nanchong 637000,China
2.Senior Department of Otolaryngology-head & Neck Surgery,the Sixth Medical Center of PLA General Hospital,Beijing 100853,China
-
Received:2024-02-20Accepted:2024-06-04Online:2024-09-25Published:2024-10-22 -
Contact:Ning YU
氘及其标记化合物在生物医药研究中的应用进展
- 1.川北医学院,四川 南充 637000
2.解放军总医院第六医学中心耳鼻咽喉头颈外科医学部,北京 100853
-
通讯作者:于宁 -
作者简介:唐金格 E-mail: tangjinge6@163.com; -
基金资助:国家重点研发项目(2020YFC2004001);北京市自然科学基金项目(7222185)
CLC Number:
Cite this article
Jinge TANG, Wei REN, Qingqing JIANG, Ning YU. Progress in the Application of Deuterium and its Labeled Compounds in Biomedical Research[J]. Current Biotechnology, 2024, 14(5): 785-792.
唐金格, 任巍, 蒋晴晴, 于宁. 氘及其标记化合物在生物医药研究中的应用进展[J]. 生物技术进展, 2024, 14(5): 785-792.
share this article
| 1 | VOGES R, HEYS J, MOENIUS T. Preparation of tritium‐labelled compounds by isotope exchange reactions[M]. German:Wiley, 2009, 47-107. |
| 2 | ATZRODT J, DERDAU V, KERR W J, et al.. Deuterium- and tritium-labelled compounds: applications in the life sciences[J]. Angew. Chem. Int. Ed., 2018, 57(7): 1758-1784. |
| 3 | YANG H, HESK D. Base metal-catalyzed hydrogen isotope exchange[J]. J. Label. Compd. Radiopharm., 2020, 63(6): 296-307. |
| 4 | DI GIUSEPPE A, CASTARLENAS R, ORO L A. Mechanistic considerations on catalytic H/D exchange mediated by organometallic transition metal complexes[J]. C. R. Chim., 2015, 18(7): 713-741. |
| 5 | LIU M, CHEN X, CHEN T, et al.. A facile and general acid-catalyzed deuteration at methyl groups of N-heteroarylmethanes[J]. Org. Biomol. Chem., 2017, 15(12): 2507-2511. |
| 6 | SAJIKI H, SAWAMA Y, MONGUCHI Y. Efficient H-D exchange reactions using heterogeneous platinum-group metal on carbon-H2-D2O system[J]. Synlett, 2012, 23(7): 959-972. |
| 7 | CHATTERJEE B, KRISHNAKUMAR V, GUNANATHAN C. Selective α‐deuteration of amines and amino acids using D2O[J]. Org. Lett., 2016, 18(22): 5892‐5895. |
| 8 | LEGROS F, FERNANDEZ-RODRIGUEZ P, MISHRA A, et al.. Photoredox-mediated hydrogen isotope exchange reactions of amino-acids, peptides, and peptide-derived drugs[J]. Chemistry, 26(56): 12738-12742. |
| 9 | LOH Y Y, NAGAO K, HOOVER A J, et al.. Photoredox-catalyzed deuteration and tritiation of pharmaceutical compounds[J]. Science, 2017, 358(6367): 1182-1187. |
| 10 | KOPF S, BOURRIQUEN F, LI W, et al.. Recent developments for the deuterium and tritium labeling of organic molecules[J]. Chem. Rev., 2022, 122(6): 6634-6718. |
| 11 | LIU C, CHEN Z, SU C, et al.. Controllable deuteration of halogenated compounds by photocatalytic D2O splitting[J/OL]. Nat. Commun., 2018, 9(1): 80[2024-07-22]. . |
| 12 | GOU X Y, LI Y, WANG X G, et al.. Ruthenium-catalyzed ortho-selective CAr-H amination of heteroaryl arenes with di-tert-butyldiaziridinone[J]. Chem. Commun., 2019, 55(38): 5487-5490. |
| 13 | RUIZ-CASTANEDA M, CARRIÓN M C, SANTOS L, et al.. A biphasic medium slows down the transfer hydrogenation and allows a selective catalytic deuterium labeling of amines from imines mediated by a Ru-H/D+ exchange in D2O[J]. ChemCatChem, 2018,10(23): 5541-5550. |
| 14 | COOK A, PRAKASH S, ZHENG Y L, et al.. Exhaustive reduction of esters enabled by nickel catalysis[J]. J. Am. Chem. Soc., 2020, 142(18): 8109-8115. |
| 15 | FENG K, QUEVEDO R E, KOHRT J T, et al.. Late-stage oxidative C(sp3)-H methylation[J]. Nature, 2020, 580(7805): 621-627. |
| 16 | SKLYARUK J, BORGHS J C, EL-SEPELGY O, et al.. Catalytic C-1 alkylation with methanol and isotope-labeled methanol[J]. Angew. Chem. Int. Edit., 2019, 58(3): 775-779. |
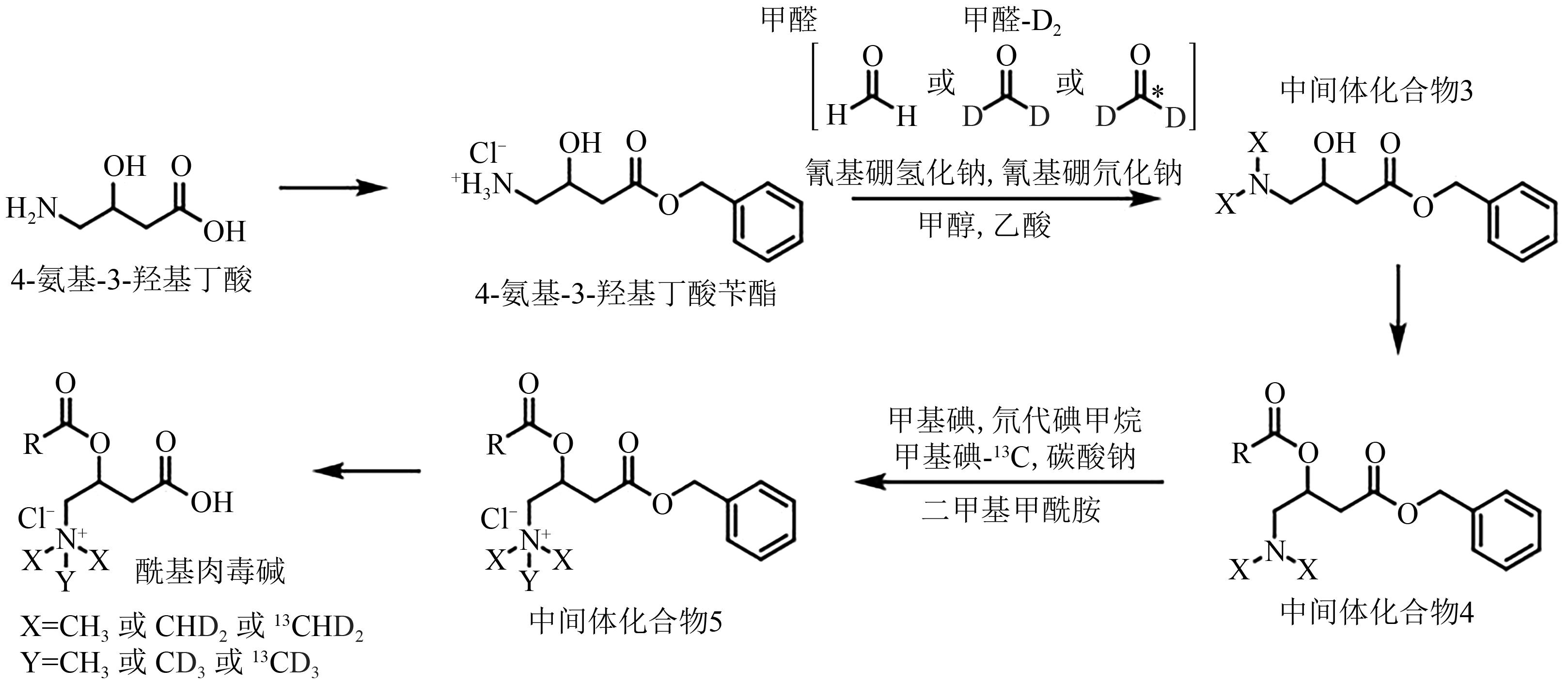
| 17 | DAI X, LV C, SUN J, et al.. A facile synthesis of isotope labeled acylcarnitines[J]. J. Label. Compd. Radiopharm., 2021, 64(5): 217-224. |
| 18 | HESK D, KOHARSKI D, MCNAMARA P, et al.. Synthesis of 3H, 13C2, 2H4 14C-SCH 430765 and 35S-SCH 500946, potent and selective inhibitors of the NPY5 receptor[J]. J. Label. Compd. Radiopharm., 2018, 61(7): 533-539. |
| 19 | WILKINSON D J, BROOK M S, SMITH K, et al.. Stable isotope tracers and exercise physiology: past, present and future[J]. J. Physiol., 2017, 595(9): 2873-2882. |
| 20 | WEIS D. Hydrogen exchange mass spectrometry of proteins: fundamentals, methods, and applications[M]. New York: John Wiley&Sons, 2016. |
| 21 | JAMES E I, MURPHREE T A, VORAUER C, et al.. Advances in hydrogen/deuterium exchange mass spectrometry and the pursuit of challenging biological systems[J]. Chem. Rev., 2022, 122(8): 7562-7623. |
| 22 | DENG B, LENTO C, WILSON D J. Hydrogen deuterium exchange mass spectrometry in biopharmaceutical discovery and development-a review[J]. Anal. Chim. Acta, 2016, 940: 8-20. |
| 23 | WALLS A C, FIALA B, SCHÄFER A, et al.. Elicitation of potent neutralizing antibody responses by designed protein nanoparticle vaccines for SARS-CoV-2[J]. Cell, 2020, 183(5): 1367-1382. |
| 24 | PANTAZATOS D, KIM J S, KLOCK H E, et al.. Rapid refinement of crystallographic protein construct definition employing enhanced hydrogen/deuterium exchange MS[J]. Proc. Natl. Acad. Sci. USA, 2004, 101(3): 751-756. |
| 25 | MASSON G R, BURKE J E, AHN N G, et al.. Recommendations for performing, interpreting and reporting hydrogen deuterium exchange mass spectrometry (HDX-MS) experiments[J]. Nat. Meth., 2019, 16(7): 595-602. |
| 26 | IACOB R E, KRYSTEK S R, HUANG R Y, et al.. Hydrogen/deuterium exchange mass spectrometry applied to IL-23 interaction characteristics: potential impact for therapeutics[J]. Expert Rev. Proteom., 2015, 12(2): 159-169. |
| 27 | POLVOY I, QIN H, FLAVELL R R, et al.. Deuterium metabolic imaging-rediscovery of a spectroscopic tool[J/OL]. Metabolites, 2021, 11(9): 570[2024-07-22]. . |
| 28 | KAGGIE J D, KHAN A S, MATYS T, et al.. Deuterium metabolic imaging and hyperpolarized 13C-MRI of the normal human brain at clinical field strength reveals differential cerebral metabolism[J/OL]. NeuroImage, 2022, 257: 119284[2024-07-22]. . |
| 29 | MAJZNER K, TOTT S, ROUSSILLE L, et al.. Uptake of fatty acids by a single endothelial cell investigated by Raman spectroscopy supported by AFM[J]. Analyst, 2018, 143(4): 970-980. |
| 30 | HARTMANN B, MÜLLER M, SEYLER L, et al.. Feasibility of deuterium magnetic resonance spectroscopy of 3-O-methylglucose at 7 Tesla[J/OL]. PLoS One, 2021, 16(6): e0252935[2024-07-22]. . |
| 31 | FLOCKE V, TEMME S, BOUVAIN P, et al.. Noninvasive assessment of metabolic turnover during inflammation by in vivo deuterium magnetic resonance spectroscopy[J/OL]. Front. Immunol., 2023, 14: 1258027[2024-07-22]. . |
| 32 | HARRIS J J, JOLIVET R, ATTWELL D. Synaptic energy use and supply[J]. Neuron, 2012, 75(5): 762-777. |
| 33 | LU M, ZHU X H, ZHANG Y, et al.. Quantitative assessment of brain glucose metabolic rates using in vivo deuterium magnetic resonance spectroscopy[J]. J. Cereb. Blood Flow Metab., 2017, 37(11): 3518-3530. |
| 34 | DE FEYTER H M, BEHAR K L, CORBIN Z A, et al.. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo [J/OL]. Sci. Adv., 2018, 4(8): eaat7314[2024-07-22]. . |
| 35 | WADA Y, SATO Y, MIYAZAKI K, et al.. The reduced/oxidized state of plasma albumin is modulated by dietary protein intake partly via albumin synthesis rate in rats[J]. Nutr. Res., 2017, 37: 46-57. |
| 36 | 田颖,孟婵芳,时明慧,等.重水标记法测定大鼠血浆清蛋白合成动力的研究[J].肠外与肠内营养,2018,25(1):52-55+61. |
| TIAN Y, MENG C F, SHI M H, et al.. Kinetic parameters of plasma albumin synthesis by deuterated water[J]. Parenteral & Enteral Nutri.,2018, 25(1): 52-55+61. | |
| 37 | LARA R, SECKL M J, PARDO O E. The p90 RSK family members: common functions and isoform specificity[J]. Cancer Res., 2013, 73(17): 5301-5308. |
| 38 | CHRYSOSTOMOU S, ROY R, PRISCHI F, et al.. Repurposed floxacins targeting RSK4 prevent chemoresistance and metastasis in lung and bladder cancer[J/OL]. Sci. Transl. Med., 2021, 13(602): eaba4627[2024-07-22]. . |
| 39 | WOJCIK J, HANTSCHEL O, GREBIEN F, et al.. A potent and highly specific FN3 monobody inhibitor of the Abl SH2 domain[J]. Nat. Struct. Mol. Biol., 2010, 17(4): 519-527. |
| 40 | LANKFORD C S, FRUCHT D M. A unique role for IL-23 in promoting cellular immunity[J]. J. Leukoc. Biol., 2003, 73(1): 49-56. |
| 41 | 赵鹏翔,谢飞,刘梦昱,等.氢气生物医学研究进展[J].生物技术进展,2021,11(4):503-517. |
| ZHAO P X, XIE F, LIU M Y, et al.. Research progress in hydrogen biomedical science[J]. Curr. Biotechnol., 2021, 11(4): 503-517. | |
| 42 | HYSPLER R, TICHA A, SCHIERBEEK H, et al.. The evaluation and quantitation of dihydrogen metabolism using deuterium isotope in rats[J/OL]. PLoS ONE, 2015, 10(6): e0130687[2024-07-22]. . |
| 43 | DI MARTINO R M C, MAXWELL B D, PIRALI T. Deuterium in drug discovery: progress, opportunities and challenges[J]. Nat. Rev. Drug Discov., 2023, 22(7): 562-584. |
| 44 | SCHMIDT C. First deuterated drug approved[J]. Nat. Biotechnol., 2017, 35(6): 493-494. |
| 45 | SCOTT-STEVENS P, ATACK J R, SOHAL B, et al.. Rodent pharmacokinetics and receptor occupancy of the GABAA receptor subtype selective benzodiazepine site ligand L-838417[J]. Biopharm. Drug Dispos., 2005, 26(1): 13-20. |
| 46 | BRAMAN V, LIU J F, HARBESON S, et al.. Preliminary clinical outcomes for CTP-354, a novel subtype-selective GABA(A) modulator[J]. Ann. Neurol., 2014, 76(S18): 104-104. |
| 47 | HARBESON S L, MORGAN A J, LIU J F, et al.. Altering metabolic profiles of drugs by precision deuteration 2: discovery of a deuterated analog of ivacaftor with differentiated pharmacokinetics for clinical development[J]. J. Pharmacol. Exp. Ther., 2017, 362(2): 359-367. |
| 48 | PIRALI T, SERAFINI M, CARGNIN S, et al.. Applications of deuterium in medicinal chemistry[J]. J. Med. Chem., 2019, 62(11): 5276-5297. |
| 49 | CALINSKI D M, ZHANG H, LUDEMAN S, et al.. Hydroxylation and N-dechloroethylation of Ifosfamide and deuterated ifosfamide by the human cytochrome P450s and their commonly occurring polymorphisms[J]. Drug Metab. Dispos. Biol. Fate Chem., 2015, 43(7): 1084-1090. |
| 50 | FRAM D M, ALMENOFF J S, DUMOUCHEL W. Empirical Bayesian data mining for discovering patterns in post-marketing drug safety[C]//The Ninth ACM SIGKDD International Conference, ACM, 2003. |
| 51 | UTTAMSINGH V, GALLEGOS R, LIU J F, et al.. Altering metabolic profiles of drugs by precision deuteration: reducing mechanism-based inhibition of CYP2D6 by paroxetine[J]. J. Pharmacol. Exp. Ther., 2015, 354(1): 43-54. |
| 52 | RODRIGUEZ-VIEITEZ E, CARTER S F, CHIOTIS K, et al.. Comparison of early-phase 11C-deuterium-l-deprenyl and 11C-pittsburgh compound B PET for assessing brain perfusion in alzheimer disease[J]. J. Nucl. Med., 2016, 57(7): 1071-1077. |
| 53 | FOWLER J S, WANG G J, LOGAN J, et al.. Selective reduction of radiotracer trapping by deuterium substitution: comparison of carbon-11-L-deprenyl and carbon-11-deprenyl-D2 for MAO B mapping[J]. J. Nucl. Med., 1995, 36(7): 1255-1262. |
| 54 | DEGRADO T R, COLEMAN R E, WANG S, et al.. Synthesis and evaluation of 18F-labeled choline as an oncologic tracer for positron emission tomography: initial findings in prostate cancer[J]. Cancer Res., 2001, 61(1): 110-117. |
| 55 | BANSAL A, WANG S, HARA T, et al.. Biodisposition and metabolism of [(18)F] fluorocholine in 9L glioma cells and 9L glioma-bearing fisher rats[J]. Eur. J. Nucl. Med. Mol., 2008, 35(6): 1192-1203. |
| [1] | Shuai SHI, Yingchun ZHAO, Ruihong LIN, Wengang LI, Shuo WANG, Jing YU. Application of Hot Melt Extrusion Technology in Biomedicine Field Based on Patent Data [J]. Current Biotechnology, 2024, 14(2): 331-337. |
| [2] | Ruixue SUN, Wei MI, Zihong YE. Research Advances in Protein Interactions Based on Mass Spectrometry [J]. Current Biotechnology, 2022, 12(2): 161-167. |
| [3] | QIN Haibo1, ZHU Jianming2*. Progress on Environmental Geochemistry of Selenium in Typical High-Se Areas in China [J]. Curr. Biotech., 2017, 7(5): 367-373. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||