


生物技术进展 ›› 2023, Vol. 13 ›› Issue (5): 785-797.DOI: 10.19586/j.2095-2341.2023.0058
张晓旭1( ), 肖向茜1,2, 潘逸群1, 顾烨翔1, 董礼1, 党浩然1, 康茜1, 王明连1,2(
), 肖向茜1,2, 潘逸群1, 顾烨翔1, 董礼1, 党浩然1, 康茜1, 王明连1,2( )
)
收稿日期:2023-04-19
接受日期:2023-05-18
出版日期:2023-09-25
发布日期:2023-10-10
通讯作者:
王明连
作者简介:张晓旭 E-mail: 18332756281@163.com;
基金资助:
Xiaoxu ZHANG1( ), Xiangqian XIAO1,2, Yiqun PAN1, Yexiang GU1, Li DONG1, Haoran DANG1, Xi KANG1, Minglian WANG1,2(
), Xiangqian XIAO1,2, Yiqun PAN1, Yexiang GU1, Li DONG1, Haoran DANG1, Xi KANG1, Minglian WANG1,2( )
)
Received:2023-04-19
Accepted:2023-05-18
Online:2023-09-25
Published:2023-10-10
Contact:
Minglian WANG
摘要:
基于石墨烯的生物相容性和对单链核酸的吸附性,研究烯壳铁氮磁珠(graphene-shelled ferro-nitride magnetic beads, GFeNMB)运载靶向Survivin的反义寡核苷酸(antisense oligonucleotide, ASO)及其对肿瘤细胞的抑制作用。首先用RNA Draw针对Survivin mRNA的二级结构设计ASO,合成FAM标记和未标记的ASO;基于石墨烯对单链核酸的吸附性和对荧光团的淬灭性,采用酶标仪检测荧光强度方法检测GFeNMB对ASO的吸附能力,并对GFeNMB和GFeNMB-ASO表征;磁极作用下将Survivin ASO磁转染至人非小细胞肺癌细胞A549中,荧光显微镜观察GFeNMB将ASO载入细胞的能力;ASO转染后,采用Western blot检测Survivinr的表达,活性氧(reactive oxygen species, ROS)试剂盒、流式细胞仪检测细胞凋亡,CCK-8和划痕实验检测细胞增殖和迁移能力。结果表明,GFeNMB对ASO具有良好的吸附性,GFeNMB与ASO混合20 min达最大吸附量(0.44 μg·mg-1);FAM-ASO经磁性载入细胞内呈绿色荧光且集中于细胞核,GFeNMB介导的核转染能力显著优于脂质体;ASO经磁转染至细胞后,Survivin蛋白的降低水平优于未处理组和脂质体转染组;磁转染Survivin ASO后,肿瘤细胞凋亡比例增大,细胞内ROS升高,细胞增殖和迁移能力受到抑制。综上,GFeNMB可将Survivin ASO转染至肿瘤细胞中,能够抑制细胞增殖,诱导细胞凋亡。GFeNMB-ASO磁转染细胞后下调靶基因表达的情况表明,GFeNMB有望成为单链寡核苷酸的转染介质。
中图分类号:
张晓旭, 肖向茜, 潘逸群, 顾烨翔, 董礼, 党浩然, 康茜, 王明连. 烯壳铁氮磁珠介导Survivin ASO对肿瘤细胞的转染和抑制作用[J]. 生物技术进展, 2023, 13(5): 785-797.
Xiaoxu ZHANG, Xiangqian XIAO, Yiqun PAN, Yexiang GU, Li DONG, Haoran DANG, Xi KANG, Minglian WANG. Transfection and Inhibition of Tumor Cells by Survivin ASO Mediated by Graphene-shelled Ferro-nitride Magnetic Beads[J]. Current Biotechnology, 2023, 13(5): 785-797.
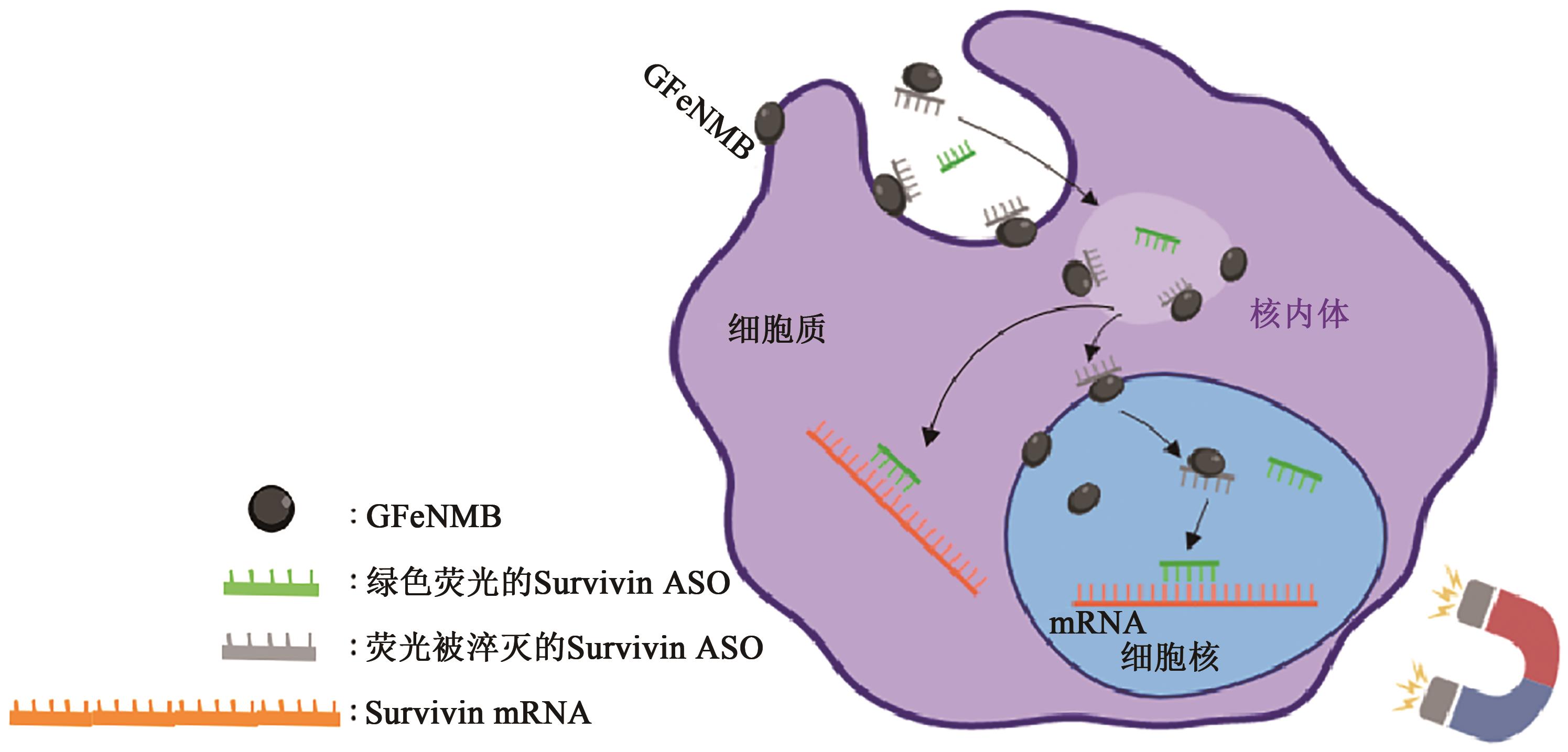
图3 磁转染原理磁转染:基于GFeNMB外壳石墨烯的吸附性使ASO能与GFeNMB结合形成药物载体,此时FAM修饰的ASO荧光被猝灭。将药物载体加入培养的肿瘤细胞中,然后细胞培养板置于磁铁上,在培养箱中孵育40 min。磁场可以使药物载体快速沉积在转染细胞上,实现核酸的高效递送。转染数小时后,由于ASO与survivin mRNA交杂,荧光恢复。
Fig. 3 General mechanism of magnetofection
| 寡核苷酸 | 序列(5'→3') | Survivin mRNA上的靶位 |
|---|---|---|
| ASO1[ | 5'-CCCAGCCTTCCAGCTCCTTG-3' | 232~251 |
| ASO2 | 5'-CTCTATGGGGTCGTCATCTG-3' | 255~274 |
| ASO3 | 5'-TCTTGAATGTAGAGATGCGG-3' | 100~119 |
| ASO4 | 5'-CAAATCCATCATCTTACGCC-3' | 1 491~1 510 |
表1 反义寡核苷酸序列及其在Survivin mRNA上的靶位
Table 1 Sequences of antisense oligonucletides and their target sites on the Survivin mRNA
| 寡核苷酸 | 序列(5'→3') | Survivin mRNA上的靶位 |
|---|---|---|
| ASO1[ | 5'-CCCAGCCTTCCAGCTCCTTG-3' | 232~251 |
| ASO2 | 5'-CTCTATGGGGTCGTCATCTG-3' | 255~274 |
| ASO3 | 5'-TCTTGAATGTAGAGATGCGG-3' | 100~119 |
| ASO4 | 5'-CAAATCCATCATCTTACGCC-3' | 1 491~1 510 |
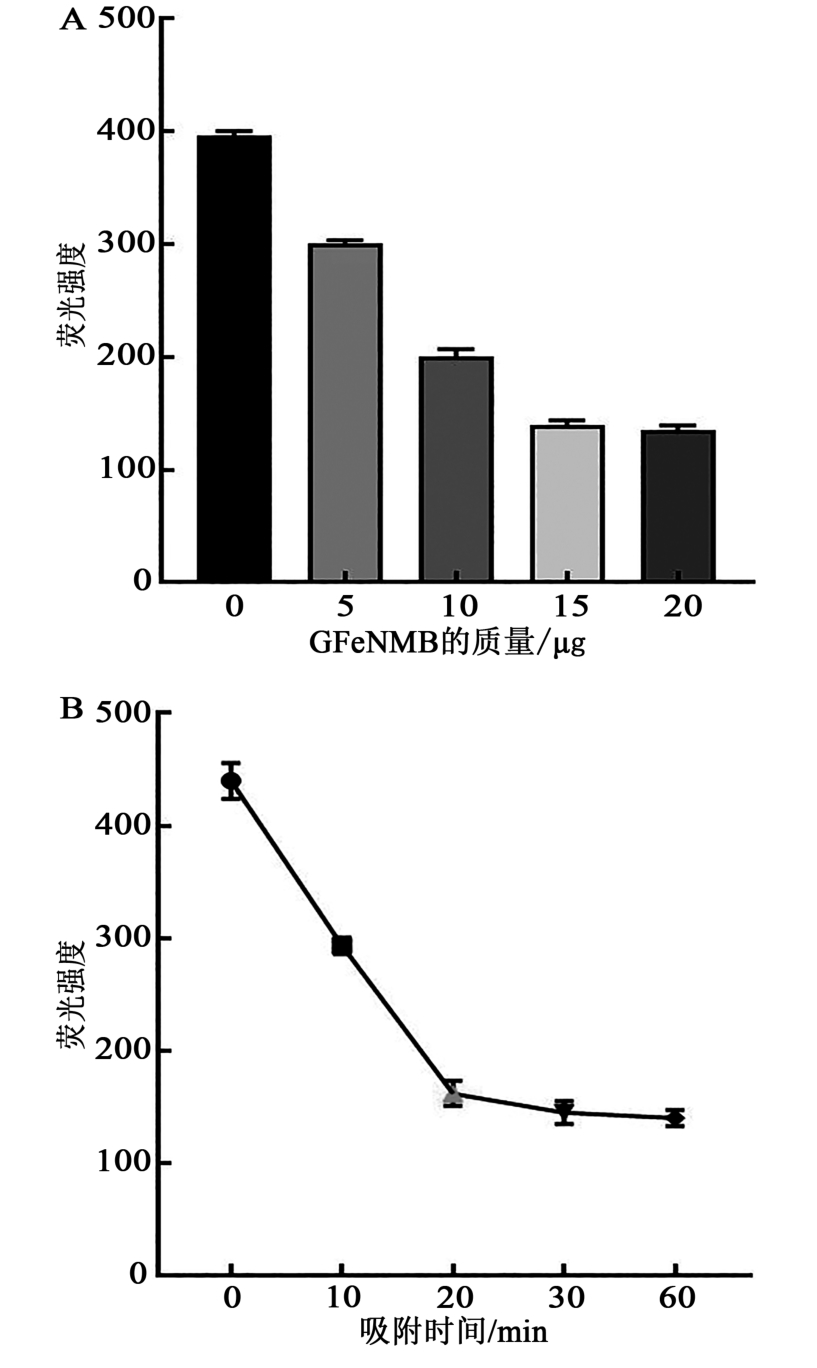
图4 GFeNMB吸附Survivin ASO的质量和时间关系A:不同质量GFeNMB吸附Survivin ASO后剩余液体的荧光强度;B:不同时间GFeNMB吸附Survivin ASO后剩余液体的荧光强度
Fig. 4 Quality and time relationship of Survivin ASO adsorbed by GFeNMB
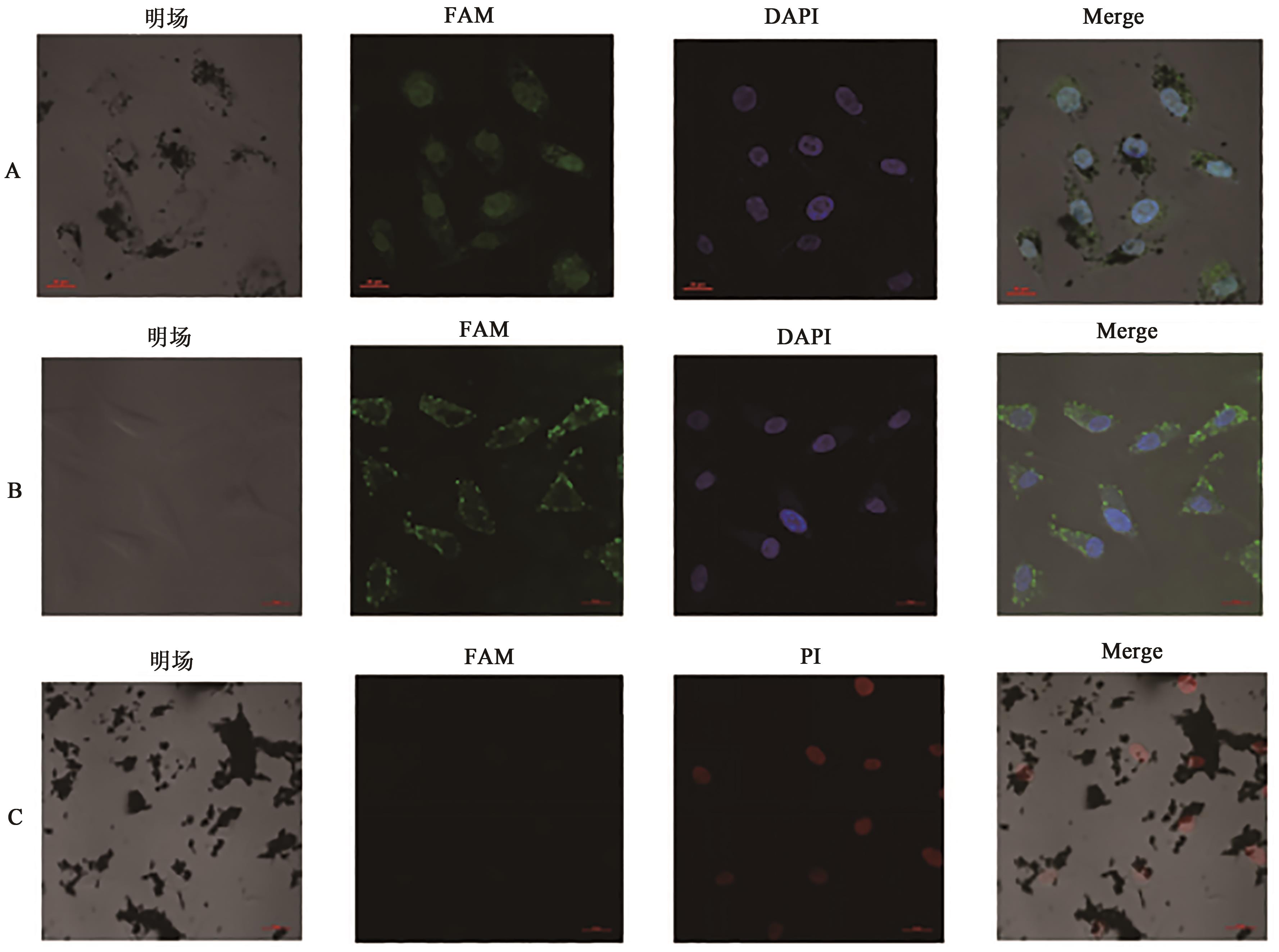
图8 GFeNMB-Survivin ASO转染后细胞的共聚焦显微镜荧光结果A: GFeNMB-Survivin ASO转染A549细胞; B: Lip-Survivin ASO转染A549细胞; C: GFeNMB-Survivin ASO转染A549细胞后48 h。
Fig. 8 Fluorescence images of cells after GFeNMB-Survivin ASO transfection
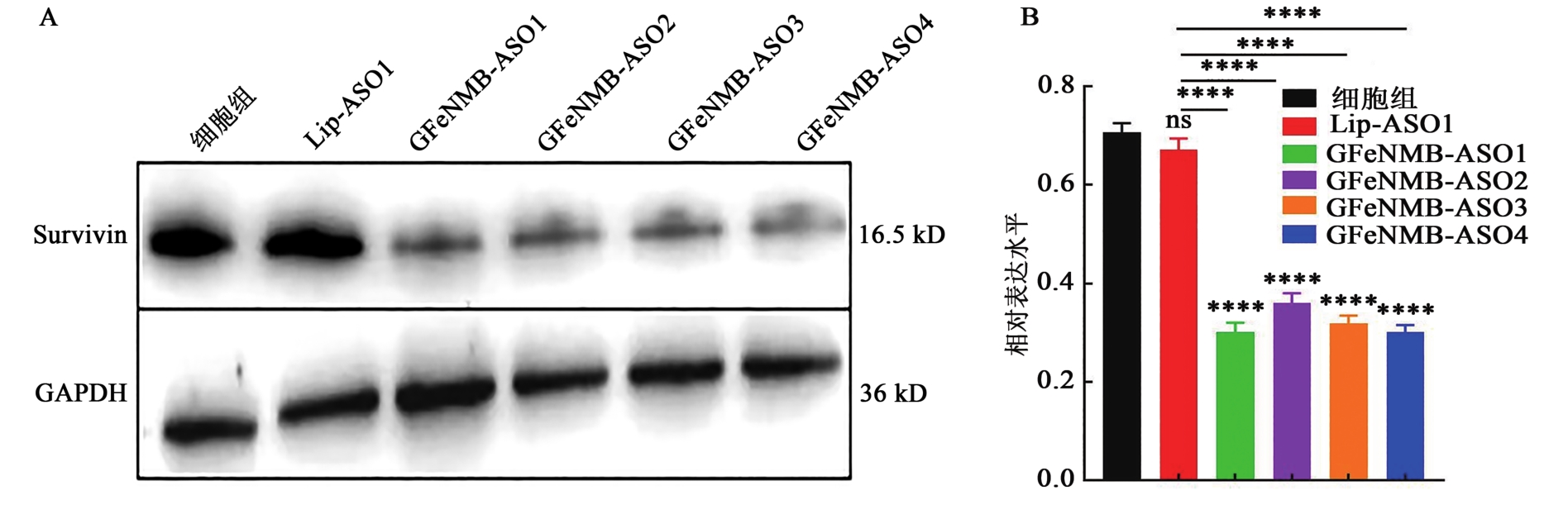
图9 GFeNMB-Survivin ASO和Lip-Survivin ASO转染后Western blot检测蛋白表达A: Western blot检测各组细胞中Survivin的蛋白表达; B: Western blot灰度分析。ns表示与细胞组和脂质体组相比无显著性差异,****表示与细胞组和脂质体组相比差异在P<0.000 1水平有统计学意义。
Fig. 9 Protein expression of GFeNMB-Survivin ASO and Lip-Survivin ASO transfected by Western blot
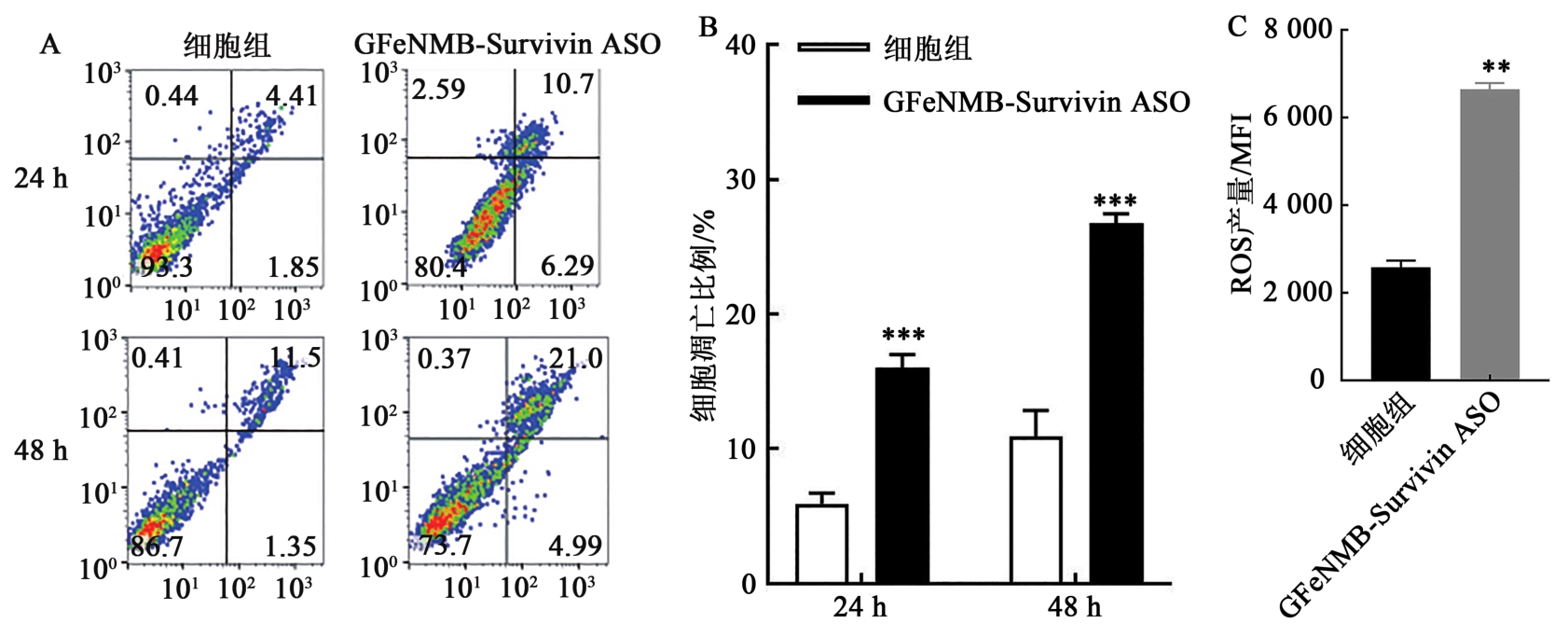
图10 GFeNMB-Survivin ASO转染后细胞凋亡情况A:流式细胞仪检测细胞组和实验组的细胞凋亡;B:转染后24、48 h凋亡细胞比;C:GFeNMB-Survivin ASO转染后细胞内ROS水平。**、***分别表示与细胞对照组相比,差异在P<0.01、P<0.001水平有统计学意义。
Fig. 10 Apoptosis of cells after transfection with GFeNMB-Survivin ASO
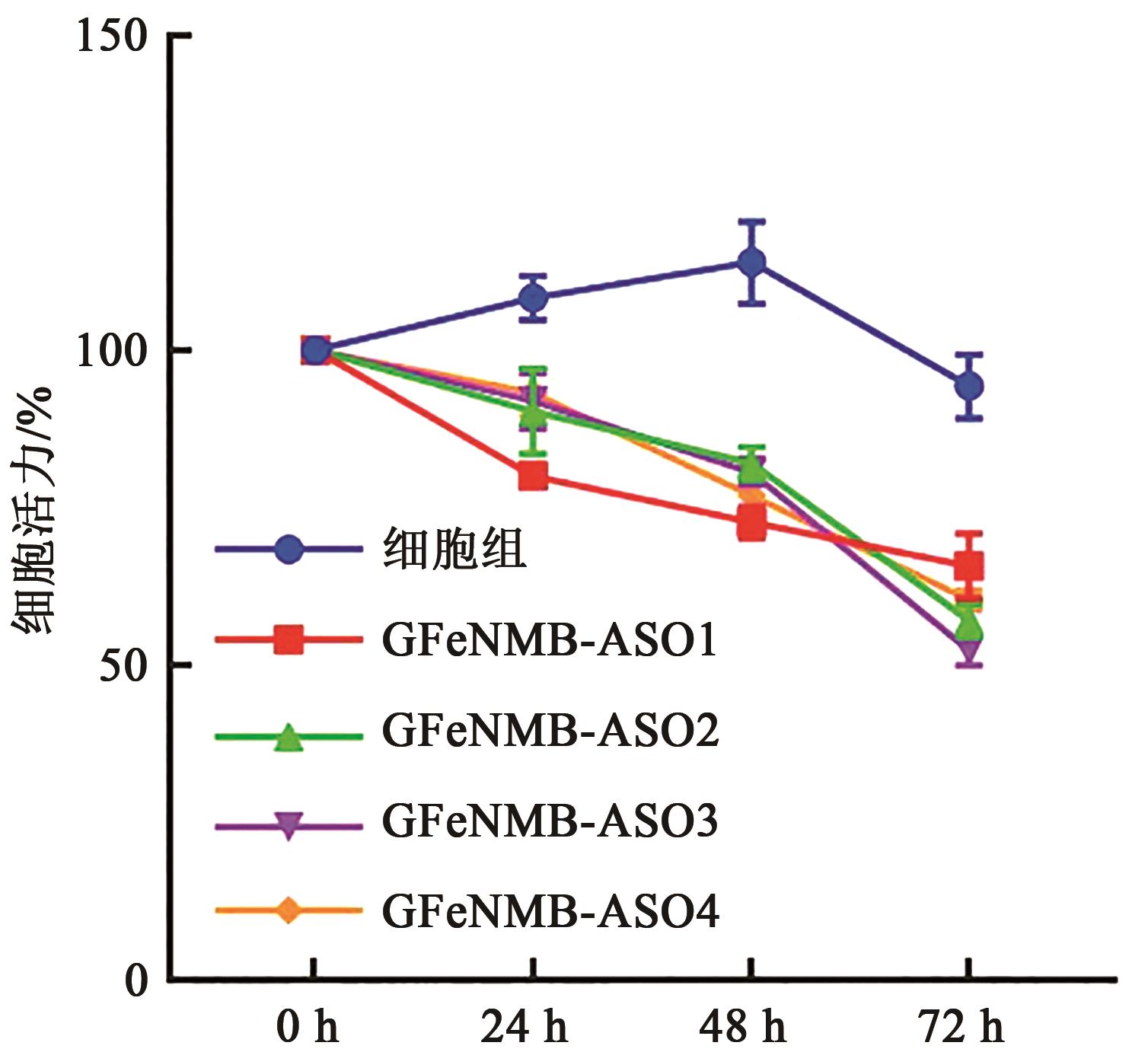
图11 不同时间段不同序列GFeNMB-Survivin ASO转染后细胞活力
Fig. 11 Cell viability after transfection with GFeNMB-Survivin ASO with different sequences at different periods
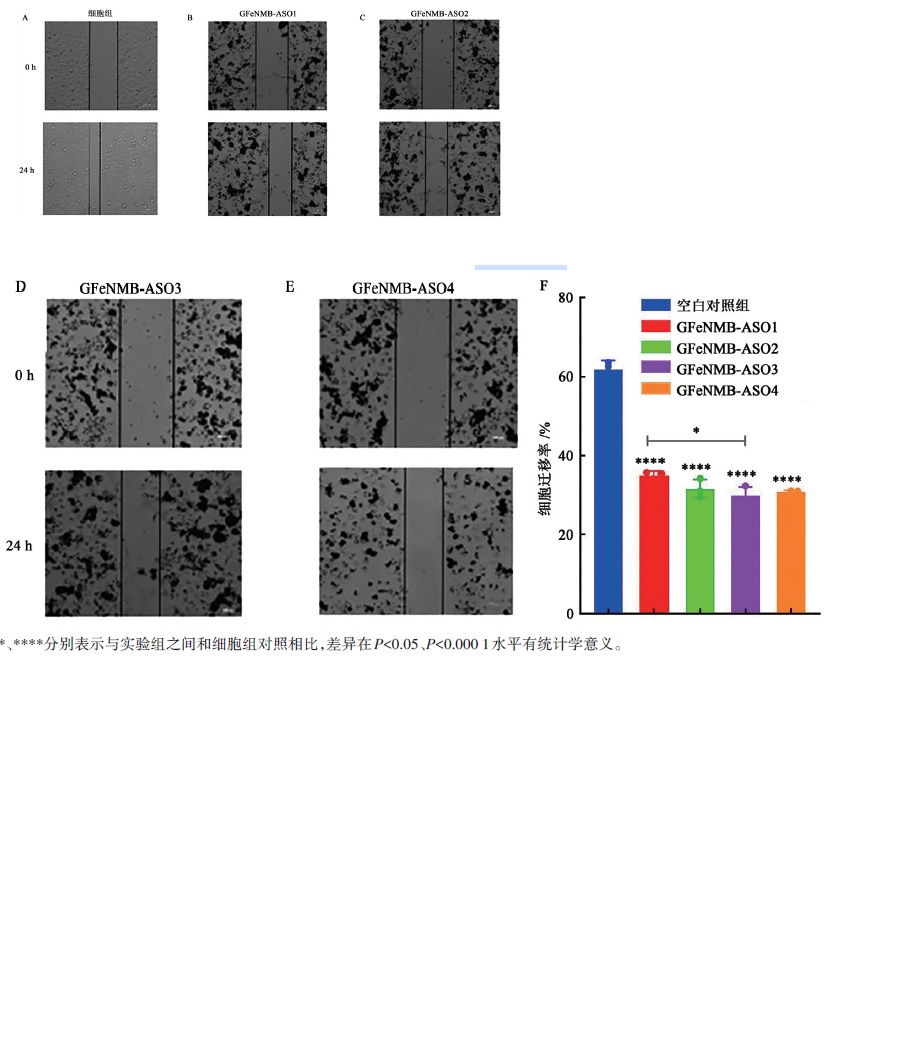
图12 GFeNMB-Survivin ASO转染后划痕实验在0、24 h细胞迁移结果注:*、****分别表示与实验组之间和细胞组对照相比,差异在P<0.05、P<0.000 1水平有统计学意义。
Fig. 12 Cell migration results after transfected by GFeNMB-Survivin ASO at 0 and 24 h
| 1 | SUNG H, FERLAY J, SIEGEL R L, et al.. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J. Clin., 2021, 71(3): 209-249. |
| 2 | JOLLY P, ESTRELA P, LADOMERY M. Oligonucleotide-based systems: DNA, microRNAs, DNA/RNA aptamers[J]. Essays Biochem., 2016, 60(1): 27-35. |
| 3 | ALHAMADANI F, ZHANG K, PARIKH R, et al.. Adverse drug reactions and toxicity of the food and drug administration-approved antisense oligonucleotide drugs[J]. Drug Metab. Dispos., 2022, 50(6): 879-887. |
| 4 | BLOKPOEL F L A, CHAN S Y, VAZQUEZ REINA S, et al.. Rapidly transducing and spatially localized magnetofection using peptide-mediated non-viral gene delivery based on iron oxide nanoparticles[J]. ACS Appl. Nano Mater., 2021, 4(1): 167-181. |
| 5 | PEERY R C, LIU J Y, ZHANG J T. Targeting survivin for therapeutic discovery: past, present, and future promises[J]. Drug Discov. Today, 2017, 22(10): 1466-1477. |
| 6 | SHOJAEI F, YAZDANI-NAFCHI F, BANITALEBI-DEHKORDI M, et al.. Trace of survivin in cancer[J]. Eur. J. Cancer Prev., 2019, 28(4): 365-372. |
| 7 | LI Y, LU W, YANG J, et al.. Survivin as a biological biomarker for diagnosis and therapy[J]. Expert Opin. Biol. Ther., 2021, 21(11): 1429-1441. |
| 8 | MOBAHAT M, NARENDRAN A, RIABOWOL K. Survivin as a preferential target for cancer therapy[J]. Int. J. Mol. Sci., 2014, 15(2): 2494-2516. |
| 9 | TAMM I, WANG Y, SAUSVILLE E, et al.. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs[J]. Cancer Res., 1998, 58(23): 5315-5320. |
| 10 | RAMASAMY T, RUTTALA H B, MUNUSAMY S, et al.. Nano drug delivery systems for antisense oligonucleotides (ASO) therapeutics[J]. J. Control. Release, 2022, 352: 861-878. |
| 11 | MANTZ A, PANNIER A K. Biomaterial substrate modifications that influence cell-material interactions to prime cellular responses to nonviral gene delivery[J]. Exp. Biol. Med., 2019, 244(2): 100-113. |
| 12 | SHIM G, KIM D, LE Q V, et al.. Nonviral delivery systems for cancer gene therapy: strategies and challenges[J]. Curr. Gene Ther., 2018, 18(1): 3-20. |
| 13 | JIAO Y, XIA Z L, ZE L J, et al.. Research progress of nucleic acid delivery vectors for gene therapy[J/OL]. Biomed. Microdevices, 2020, 22(1): 16[2022-10-10]. . |
| 14 | ZU H, GAO D. Non-viral vectors in gene therapy: recent development, challenges, and prospects[J/OL]. AAPS J., 2021, 23(4): 78[2022-10-13]. . |
| 15 | WEI B, JIN X, WANG Q, et al. Synthesis of carbon coated iron nitride nanoparticles by using microwave plasma technique[J/OL]. Mater. Res. Express, 2020, 7(9): 096103[2021-05-06]. . |
| 16 | WU Z, DENG W, ZHOU W, et al.. Novel magnetic polysaccharide/graphene oxide @Fe3O4 gel beads for adsorbing heavy metal ions[J]. Carbohydr. Polym., 2019, 216: 119-128. |
| 17 | LI J, WANG M, JIA R, et al.. Graphene-coated iron nitride streptavidin magnetic beads: preparation and application in SARS-CoV-2 enrichment[J/OL]. Magnetochemistry, 2022: 8[2022-10-12]. . |
| 18 | XU Z, LEI X, TU Y, et al.. Dynamic cooperation of hydrogen binding and π stacking in ssDNA adsorption on graphene oxide[J]. Chemistry, 2017, 23(53): 13100-13104. |
| 19 | LEI X, MA H, FANG H. Length feature of ssDNA adsorption onto graphene oxide with both large unoxidized and oxidized regions[J]. Nanoscale, 2020, 12(12): 6699-6707. |
| 20 | ZENG S, CHEN L, WANG Y, et al.. Exploration on the mechanism of DNA adsorption on graphene and graphene oxide via molecular simulations[J/OL]. J. Phys. D Appl. Phys., 2015, 48(27):275402[2021-11-10]. . |
| 21 | IWE I, LI Z, HUANG J. Graphene oxide and enzyme-assisted dual-cycling amplification method for sensitive fluorometric determination of DNA[J/OL]. Mikrochim. Acta, 2019, 186(11): 716[2023-01-11]. . |
| 22 | JEONG S, KIM D M, AN S Y, et al.. Fluorometric detection of influenza viral RNA using graphene oxide[J]. Anal. Biochem., 2018, 561-562: 66-69. |
| 23 | ZHENG P, WU N. Fluorescence and sensing applications of graphene oxide and graphene quantum dots: a review[J]. Chem. Asian J., 2017, 12(18): 2343-2353. |
| 24 | LIU B, HUANG P J, KELLY E Y, et al.. Graphene oxide surface blocking agents can increase the DNA biosensor sensitivity[J]. Biotechnol. J., 2016, 11(6): 780-787. |
| 25 | PAUL T, BERA S C, AGNIHOTRI N, et al.. Single-molecule FRET studies of the hybridization mechanism during noncovalent adsorption and desorption of DNA on graphene oxide[J]. J. Phys. Chem. B, 2016, 120(45): 11628-11636. |
| 26 | LIANG L, SHEN X, ZHOU M, et al.. Theoretical evaluation of potential cytotoxicity of graphene quantum dot to adsorbed DNA[J/OL]. Materials, 2022, 15(21): 7435[2020-12-12]. . |
| 27 | MA Y, WANG J, WU J, et al.. Meta-analysis of cellular toxicity for graphene via data-mining the literature and machine learning[J/OL]. Sci. Total Environ., 2021, 793: 148532[2022-10-12]. . |
| 28 | SONG S, SHEN H, WANG Y, et al.. Biomedical application of graphene: from drug delivery, tumor therapy, to theranostics[J/OL]. Colloids Surf. B Biointerfaces, 2020, 185: 110596[2023-04-11]. . |
| 29 | SHIBATA M, KANETAKA H, FURUYA M, et al.. Cytotoxicity evaluation of iron nitride nanoparticles for biomedical applications[J]. J. Biomed. Mater. Res. A, 2021, 109(10): 1784-1791. |
| 30 | 刘庆祖, 刘建恒, 王润生, 等. 聚乙二醇化锌铁氧磁性纳米颗粒对咪喹莫特的载药性能及细胞毒性研究[J]. 解放军医学院学报, 2021, 42(4): 444-450. |
| 31 | MATZURA O, WENNBORG A. RNAdraw: an integrated program for RNA secondary structure calculation and analysis under 32-bit Microsoft Windows[J]. Comput. Appl. Biosci., 1996, 12(3): 247-249. |
| 32 | OLIE R A, SIMÕES-WÜST A P, BAUMANN B, et al.. A novel antisense oligonucleotide targeting survivin expression induces apoptosis and sensitizes lung cancer cells to chemotherapy[J]. Cancer Res., 2000, 60(11): 2805-2809. |
| 33 | LIANG T, LI J, LIU X, et al.. Preparation of CD3 antibody-conjugated, graphene oxide coated iron nitride magnetic beads and its preliminary application in T cell separation[J/OL]. Magnetochemistry, 2021: 7050058[2021-12-19]. . |
| 34 | FERENCHAK K, DEITCH I, HUCKFELDT R. Antisense oligonucleotide therapy for ophthalmic conditions[J]. Semin. Ophthalmol., 2021, 36(5-6): 452-457. |
| 35 | GHEIBI-HAYAT S M, JAMIALAHMADI K. Antisense oligonucleotide (AS-ODN) technology: principle, mechanism and challenges[J]. Biotechnol. Appl. Biochem., 2021, 68(5): 1086-1094. |
| 36 | 梁超, 徐玲, 隋雪梅. 联合运用Survivin和VEGF反义寡核苷酸对裸鼠人肺腺癌A549移植瘤的生长抑制作用[J]. 第三军医大学学报, 2013, 35(24): 2643-2647. |
| 37 | ERBA H P, SAYAR H, JUCKETT M, et al.. Safety and pharmacokinetics of the antisense oligonucleotide (ASO) LY2181308 as a single-agent or in combination with idarubicin and cytarabine in patients with refractory or relapsed acute myeloid leukemia (AML)[J]. Invest. New Drugs, 2013, 31(4): 1023-1034. |
| 38 | CARRASCO R A, STAMM N B, MARCUSSON E, et al.. Antisense inhibition of survivin expression as a cancer therapeutic[J]. Mol. Cancer Ther., 2011, 10(2): 221-232. |
| 39 | ROBERTS T C, LANGER R, WOOD M J A. Advances in oligonucleotide drug delivery[J]. Nat. Rev. Drug Discov., 2020, 19(10): 673-694. |
| [1] | 赵璐璐, 洪甜, 郝一然, 陈尔凝, 李静雯, 杜美红. 免疫磁分离技术在循环肿瘤细胞检测中的研究进展[J]. 生物技术进展, 2025, 15(4): 606-614. |
| [2] | 朱丽娜, 宋志灵. 自噬与细胞凋亡:相互作用及其在疾病中的作用[J]. 生物技术进展, 2025, 15(4): 622-626. |
| [3] | 万令飞, 潘文婷, 雍雨婷, 李元帅, 赵悦, 阎新龙. 空间转录组学在肝病研究中的应用进展[J]. 生物技术进展, 2025, 15(4): 645-654. |
| [4] | 蔡怡霏, 马叶子, 夏美娟, 刘翠翠, 王洪涛, 周家喜. 小鼠胚胎主动脉-性腺-中肾区与成年骨髓巨核细胞的分子特征分析[J]. 生物技术进展, 2025, 15(4): 702-710. |
| [5] | 梁传财, 邱波. 松果菊苷通过Nrf2/HO-1通路抑制IL-1β诱导的软骨细胞铁死亡[J]. 生物技术进展, 2025, 15(4): 720-725. |
| [6] | 刘晓雅, 张硕珉, 郑鹏, 马睿, 张朝军. DBNDD1基因在结直肠癌进展过程中的作用与机制研究[J]. 生物技术进展, 2025, 15(4): 726-734. |
| [7] | 丁瑜, 赵博, 张瑾, 高旭栋. SIRT1去乙酰化修饰调控HMGB1介导的细胞焦亡在慢性鼻窦炎伴鼻息肉中的作用[J]. 生物技术进展, 2025, 15(3): 535-543. |
| [8] | 刘乙锦, 许文扬, 魏锦骥, 宋欢喜, 李佳慧, 张丽娜. 慢病毒介导的PDE12稳定敲低和过表达胃癌细胞模型的构建及其细胞定位分析[J]. 生物技术进展, 2025, 15(2): 355-363. |
| [9] | 谭景胜, 赵官涛, 朱莉, 李玉斌. 玉米rMrh/Mrh双元转座系统体细胞转座特性分析[J]. 生物技术进展, 2024, 14(4): 601-609. |
| [10] | 段兴鹏, 刘景丽, 王澈, 尚德静. 巨噬细胞清道夫受体与Toll样受体对Ox-LDL摄取和炎症的影响[J]. 生物技术进展, 2024, 14(4): 668-675. |
| [11] | 李鸿博, 陈朱玥, 吕新星. 亚精胺缓解细胞衰老及衰老相关疾病的研究进展[J]. 生物技术进展, 2024, 14(3): 388-398. |
| [12] | 李蓓蓓, 武建强. 肿瘤细胞中FAS介导的非凋亡信号通路研究进展[J]. 生物技术进展, 2024, 14(3): 406-412. |
| [13] | 曾艳, 祝恒成, 杨康. 脱氧核糖核酸酶1在肾细胞癌中的作用及机制研究[J]. 生物技术进展, 2024, 14(3): 486-491. |
| [14] | 刘一鸣, 谢乐灵, 邢海燕, 唐克晶, 王敏, 饶青. 靶向CD74的嵌合抗原受体T细胞的抗白血病细胞作用研究[J]. 生物技术进展, 2024, 14(3): 501-505. |
| [15] | 赵维坚, 徐弘庭, 肖向茜, 盛望. 肿瘤干细胞中的Hippo信号通路研究进展[J]. 生物技术进展, 2024, 14(2): 211-220. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||